Predator-induced maternal effects determine adaptive antipredator behaviors via egg composition
- PMID: 34507981
- PMCID: PMC8449421
- DOI: 10.1073/pnas.2017063118
Predator-induced maternal effects determine adaptive antipredator behaviors via egg composition
Abstract
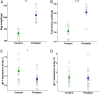
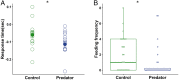
In high-risk environments with frequent predator encounters, efficient antipredator behavior is key to survival. Parental effects are a powerful mechanism to prepare offspring for coping with such environments, yet clear evidence for adaptive parental effects on offspring antipredator behaviors is missing. Rapid escape reflexes, or "C-start reflexes," are a key adaptation in fish and amphibians to escape predator strikes. We hypothesized that mothers living in high-risk environments might induce faster C-start reflexes in offspring by modifying egg composition. Here, we show that offspring of the cichlid fish Neolamprologus pulcher developed faster C-start reflexes and were more risk averse if their parents had been exposed to cues of their most dangerous natural predator during egg production. This effect was mediated by differences in egg composition. Eggs of predator-exposed mothers were heavier with higher net protein content, and the resulting offspring were heavier and had lower igf-1 gene expression than control offspring shortly after hatching. Thus, changes in egg composition can relay multiple putative pathways by which mothers can influence adaptive antipredator behaviors such as faster escape reflexes.
Keywords: C-start response; antipredator response; developmental plasticity; egg size; maternal effects.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Juvenile exposure to predator cues induces a larger egg size in fish.Proc Biol Sci. 2012 Mar 22;279(1731):1241-8. doi: 10.1098/rspb.2011.1290. Epub 2011 Oct 5. Proc Biol Sci. 2012. PMID: 21976689 Free PMC article.
-
Egg-mediated maternal effects in a cooperatively breeding cichlid fish.Sci Rep. 2023 Jun 16;13(1):9759. doi: 10.1038/s41598-023-35550-5. Sci Rep. 2023. PMID: 37328515 Free PMC article.
-
Maternal food supplementation and perceived predation risk modify egg composition and eggshell traits but not offspring condition.J Exp Biol. 2019 Oct 10;222(Pt 19):jeb201954. doi: 10.1242/jeb.201954. J Exp Biol. 2019. PMID: 31548290
-
Dropping to escape: a review of an under-appreciated antipredator defence.Biol Rev Camb Philos Soc. 2019 Apr;94(2):575-589. doi: 10.1111/brv.12466. Epub 2018 Oct 9. Biol Rev Camb Philos Soc. 2019. PMID: 30298642 Review.
-
Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness.Biol Rev Camb Philos Soc. 1994 Feb;69(1):35-59. doi: 10.1111/j.1469-185x.1994.tb01485.x. Biol Rev Camb Philos Soc. 1994. PMID: 8193216 Review.
Cited by
-
Heterogeneity in maternal mRNAs within clutches of eggs in response to thermal stress during the embryonic stage.BMC Ecol Evol. 2024 Feb 12;24(1):21. doi: 10.1186/s12862-024-02203-8. BMC Ecol Evol. 2024. PMID: 38347459 Free PMC article.
-
Transcriptomic basis of within- and trans-generational predator-induced plasticity in the freshwater snail Physa acuta.Heredity (Edinb). 2025 Jul;134(7):439-449. doi: 10.1038/s41437-025-00775-9. Epub 2025 Jun 13. Heredity (Edinb). 2025. PMID: 40514422
-
Maternal body condition and season influence RNA deposition in the oocytes of alfalfa leafcutting bees (Megachile rotundata).Front Genet. 2023 Jan 4;13:1064332. doi: 10.3389/fgene.2022.1064332. eCollection 2022. Front Genet. 2023. PMID: 36685934 Free PMC article.
-
Maternal investment and early thermal conditions affect performance and antipredator responses.Behav Ecol. 2024 Apr 26;35(4):arae035. doi: 10.1093/beheco/arae035. eCollection 2024 Jul-Aug. Behav Ecol. 2024. PMID: 38779594 Free PMC article.
-
Helping niches may trigger the development of task specialization and division of labour.Philos Trans R Soc Lond B Biol Sci. 2025 Mar 20;380(1922):20230273. doi: 10.1098/rstb.2023.0273. Epub 2025 Mar 20. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40109118 Review.
References
-
- Schwabl H., Groothuis T. G. G., “Maternal effects on behavior” in Encyclopedia of Animal Behavior, Breed M. D., Moore J., Eds. (Elsevier, 2019), pp. 483–494.
-
- Uller T., Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438 (2008). - PubMed
-
- Mousseau T. A., Fox C. W., The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (1998). - PubMed
-
- Wolf J. B., Brodie E. D. III, Cheverud J. M., Moore A. J., Wade M. J., Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 (1998). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

