A systematic review on exercise and training-based interventions for freezing of gait in Parkinson's disease
- PMID: 34508083
- PMCID: PMC8433229
- DOI: 10.1038/s41531-021-00224-4
A systematic review on exercise and training-based interventions for freezing of gait in Parkinson's disease
Abstract
Freezing of gait (FOG) in Parkinson's disease (PD) causes severe patient burden despite pharmacological management. Exercise and training are therefore advocated as important adjunct therapies. In this meta-analysis, we assess the existing evidence for such interventions to reduce FOG, and further examine which type of training helps the restoration of gait function in particular. The primary meta-analysis across 41 studies and 1838 patients revealed a favorable moderate effect size (ES = -0.37) of various training modalities for reducing subjective FOG-severity (p < 0.00001), though several interventions were not directly aimed at FOG and some included non-freezers. However, exercise and training also proved beneficial in a secondary analysis on freezers only (ES = -0.32, p = 0.007). We further revealed that dedicated training aimed at reducing FOG episodes (ES = -0.24) or ameliorating the underlying correlates of FOG (ES = -0.40) was moderately effective (p < 0.01), while generic exercises were not (ES = -0.14, p = 0.12). Relevantly, no retention effects were seen after cessation of training (ES = -0.08, p = 0.36). This review thereby supports the implementation of targeted training as a treatment for FOG with the need for long-term engagement.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
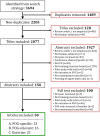
Figures










References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

