Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo
- PMID: 34508096
- PMCID: PMC8433339
- DOI: 10.1038/s41467-021-25767-1
Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo
Abstract
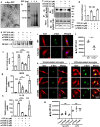
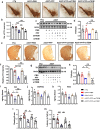
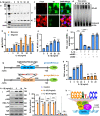
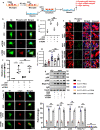
Pathways to control the spreading of α-synuclein (α-syn) and associated neuropathology in Parkinson's disease (PD), multiple system atrophy (MSA) and dementia with Lewy bodies (DLB) are unclear. Here, we show that preformed α-syn fibrils (PFF) increase the association between TLR2 and MyD88, resulting in microglial activation. The TLR2-interaction domain of MyD88 (wtTIDM) peptide-mediated selective inhibition of TLR2 reduces PFF-induced microglial inflammation in vitro. In PFF-seeded A53T mice, the nasal administration of the wtTIDM peptide, NEMO-binding domain (wtNBD) peptide, or genetic deletion of TLR2 reduces glial inflammation, decreases α-syn spreading, and protects dopaminergic neurons by inhibiting NF-κB. In summary, α-syn spreading depends on the TLR2/MyD88/NF-κB pathway and it can be reduced by nasal delivery of wtTIDM and wtNBD peptides.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

