A molecularly integrated grade for meningioma
- PMID: 34508644
- PMCID: PMC9071299
- DOI: 10.1093/neuonc/noab213
A molecularly integrated grade for meningioma
Abstract
Background: Meningiomas are the most common primary intracranial tumor in adults. Clinical care is currently guided by the World Health Organization (WHO) grade assigned to meningiomas, a 3-tiered grading system based on histopathology features, as well as extent of surgical resection. Clinical behavior, however, often fails to conform to the WHO grade. Additional prognostic information is needed to optimize patient management.
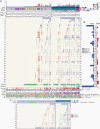
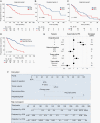
Methods: We evaluated whether chromosomal copy-number data improved prediction of time-to-recurrence for patients with meningioma who were treated with surgery, relative to the WHO schema. The models were developed using Cox proportional hazards, random survival forest, and gradient boosting in a discovery cohort of 527 meningioma patients and validated in 2 independent cohorts of 172 meningioma patients characterized by orthogonal genomic platforms.
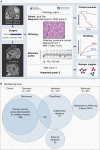
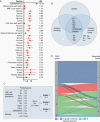
Results: We developed a 3-tiered grading scheme (Integrated Grades 1-3), which incorporated mitotic count and loss of chromosome 1p, 3p, 4, 6, 10, 14q, 18, 19, or CDKN2A. 32% of meningiomas reclassified to either a lower-risk or higher-risk Integrated Grade compared to their assigned WHO grade. The Integrated Grade more accurately identified meningioma patients at risk for recurrence, relative to the WHO grade, as determined by time-dependent area under the curve, average precision, and the Brier score.
Conclusion: We propose a molecularly integrated grading scheme for meningiomas that significantly improves upon the current WHO grading system in prediction of progression-free survival. This framework can be broadly adopted by clinicians with relative ease using widely available genomic technologies and presents an advance in the care of meningioma patients.
Keywords: copy-number alterations; meningioma.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures


References
-
- Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. - PubMed
-
- Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Revised 4th ed. IARC; 2016.
-
- Sayagués JM, Tabernero MD, Maíllo A, et al. Intratumoral patterns of clonal evolution in meningiomas as defined by multicolor interphase fluorescence in situ hybridization (FISH): is there a relationship between histopathologically benign and atypical/anaplastic lesions? J Mol Diagn. 2004;6(4):316–325. - PMC - PubMed

