Velocity of myosin-based actin sliding depends on attachment and detachment kinetics and reaches a maximum when myosin-binding sites on actin saturate
- PMID: 34508779
- PMCID: PMC8560993
- DOI: 10.1016/j.jbc.2021.101178
Velocity of myosin-based actin sliding depends on attachment and detachment kinetics and reaches a maximum when myosin-binding sites on actin saturate
Abstract
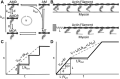
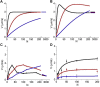
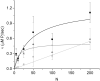
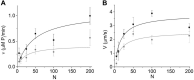
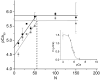
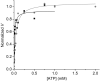
Molecular motors such as kinesin and myosin often work in groups to generate the directed movements and forces critical for many biological processes. Although much is known about how individual motors generate force and movement, surprisingly, little is known about the mechanisms underlying the macroscopic mechanics generated by multiple motors. For example, the observation that a saturating number, N, of myosin heads move an actin filament at a rate that is influenced by actin-myosin attachment and detachment kinetics is accounted for neither experimentally nor theoretically. To better understand the emergent mechanics of actin-myosin mechanochemistry, we use an in vitro motility assay to measure and correlate the N-dependence of actin sliding velocities, actin-activated ATPase activity, force generation against a mechanical load, and the calcium sensitivity of thin filament velocities. Our results show that both velocity and ATPase activity are strain dependent and that velocity becomes maximized with the saturation of myosin-binding sites on actin at a value that is 40% dependent on attachment kinetics and 60% dependent on detachment kinetics. These results support a chemical thermodynamic model for ensemble motor mechanochemistry and imply molecularly explicit mechanisms within this framework, challenging the assumption of independent force generation.
Keywords: actin; collective force; mechanics; myosin; velocity.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Bray D. 1st Ed. Garland Publishing; New York, NY: 2001. Cell Movement from Molecules to Motility.
-
- Howard J. 1st Ed. Sinauer Associates; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton.
-
- Phillips R., Kondev J., Theriot J. 1st Ed. Garland Science; New York, NY: 2010. Physical Biology of the Cell.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

