Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy - a single centre prospective study
- PMID: 34508994
- PMCID: PMC8424105
- DOI: 10.1016/j.ejca.2021.08.007
Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy - a single centre prospective study
Abstract
Aim: Patients with cancer are at an increased risk for severe coronavirus disease of 2019, thus data on the safety and efficacy of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccines are essential. We conducted this prospective study of patients with cancer vaccinated with BNT162b2 and monitored for antibody response and safety. The aim was to evaluate the rate of seropositivity and define predictors for non-reactive immune response. Furthermore, we evaluated the frequency and the severity of adverse events.
Methods: The study included patients with solid tumours undergoing anticancer treatment and immunocompetent health-care workers serving as controls. Serum titres of the receptor-binding domain (RBD) immunoglobulin G (IgG) and neutralising antibodies were measured 2-4 weeks after each vaccine dose.
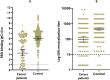
Results: The analysis included 129 patients, of which 70.5% patients were metastatic. Patients were treated with chemotherapy (55%), immunotherapy (34.1%), biological agents (24.8%), hormonal treatment (8.5%) and radiotherapy (4.6%), that were given either alone or in combinations. The seropositivity rate among patients with cancer and controls was 32.4% versus 59.8% (p < 0.0001) after the first dose and 84.1% versus 98.9% (p < 0.0001) after the second dose, respectively. Median RBD-IgG titre was lower among patients than controls (p < 0.0001). Patients who were seronegative after the second dose had significantly more comorbidities than that with patients with seropositivity (77.8% vs 41.1%, respectively, p = 0.0042).
Conclusion: Adequate antibody response after BNT162b2 vaccination was achieved after two doses but not after one dose, in patients with cancer vaccinated during anticancer therapy.
Keywords: COVID19; Cancer; Co-morbidities; IgG; Immunoglobulin; Oncology; SARS-CoV-2; Seronegativity; Seropositivity; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Figures

Comment in
-
Letter comments on: Efficacy and safety of BNT162b2 vaccination in solid cancer patients receiving anti-cancer therapy - A single-centre prospective study: Interaction between lymphopenia and anti-SARS-CoV-2 antibodies after two injections of the Pfizer-BioNTech vaccine in patients with cancers treated by chemotherapy or immunotherapy.Eur J Cancer. 2022 Jan;160:282-284. doi: 10.1016/j.ejca.2021.09.048. Epub 2021 Oct 28. Eur J Cancer. 2022. PMID: 34810045 Free PMC article. No abstract available.
-
Letter of response to comment on: Efficacy and safety of BNT162b2 vaccination in solid cancer patients receiving anti-cancer therapy - A single centre prospective study.Eur J Cancer. 2022 Jan;160:285-286. doi: 10.1016/j.ejca.2021.10.019. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840027 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

