Uncovering viral RNA-host cell interactions on a proteome-wide scale
- PMID: 34509361
- PMCID: PMC9187521
- DOI: 10.1016/j.tibs.2021.08.002
Uncovering viral RNA-host cell interactions on a proteome-wide scale
Abstract
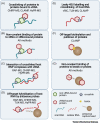
RNA viruses interact with a wide range of cellular RNA-binding proteins (RBPs) during their life cycle. The prevalence of these host-virus interactions has been highlighted by new methods that elucidate the composition of viral ribonucleoproteins (vRNPs). Applied to 11 viruses so far, these approaches have revealed hundreds of cellular RBPs that interact with viral (v)RNA in infected cells. However, consistency across methods is limited, raising questions about methodological considerations when designing and interpreting these studies. Here, we discuss these caveats and, through comparing available vRNA interactomes, describe RBPs that are consistently identified as vRNP components and outline their potential roles in infection. In summary, these novel approaches have uncovered a new universe of host-virus interactions holding great therapeutic potential.
Keywords: RNA; RNA-binding proteins; proteomics; viral ribonucleoprotein; virus; virus–host interactions.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no interests to declare.
Figures