Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder
- PMID: 34510411
- PMCID: PMC8434915
- DOI: 10.1002/14651858.CD011612.pub3
Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder
Abstract
Background: Many studies have recently been conducted to assess the antidepressant efficacy of glutamate modification in mood disorders. This is an update of a review first published in 2015 focusing on the use of glutamate receptor modulators in unipolar depression.
Objectives: To assess the effects - and review the acceptability and tolerability - of ketamine and other glutamate receptor modulators in alleviating the acute symptoms of depression in people with unipolar major depressive disorder.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Embase and PsycINFO all years to July 2020. We did not apply any restrictions to date, language or publication status.
Selection criteria: Double- or single-blinded randomised controlled trials (RCTs) comparing ketamine, memantine, esketamine or other glutamate receptor modulators with placebo (pill or saline infusion), other active psychotropic drugs, or electroconvulsive therapy (ECT) in adults with unipolar major depression.
Data collection and analysis: Three review authors independently identified studies, assessed trial quality and extracted data. The primary outcomes were response rate (50% reduction on a standardised rating scale) and adverse events. We decided a priori to measure the efficacy outcomes at different time points and run sensitivity/subgroup analyses. Risk of bias was assessed using the Cochrane tool, and certainty of the evidence was assessed using GRADE.
Main results: Thirty-one new studies were identified for inclusion in this updated review. Overall, we included 64 studies (5299 participants) on ketamine (31 trials), esketamine (9), memantine (5), lanicemine (4), D-cycloserine (2), Org26576 (2), riluzole (2), atomoxetine (1), basimglurant (1), citicoline (1), CP-101,606 (1), decoglurant (1), MK-0657 (1), N-acetylcysteine (1), rapastinel (1), and sarcosine (1). Forty-eight studies were placebo-controlled, and 48 were two-arm studies. The majority of trials defined an inclusion criterion for the severity of depressive symptoms at baseline: 29 at least moderate depression; 17 severe depression; and five mild-to-moderate depression. Nineteen studies recruited only patients with treatment-resistant depression, defined as inadequate response to at least two antidepressants. The majority of studies investigating ketamine administered as a single dose, whilst all of the included esketamine studies used a multiple dose regimen (most frequently twice a week for four weeks). Most studies looking at ketamine used intravenous administration, whilst the majority of esketamine trials used intranasal routes. The evidence suggests that ketamine may result in an increase in response and remission compared with placebo at 24 hours odds ratio (OR) 3.94, 95% confidence interval (CI) 1.54 to 10.10; n = 185, studies = 7, very low-certainty evidence). Ketamine may reduce depression rating scale scores over placebo at 24 hours, but the evidence is very uncertain (standardised mean difference (SMD) -0.87, 95% CI -1.26 to -0.48; n = 231, studies = 8, very low-certainty evidence). There was no difference in the number of participants assigned to ketamine or placebo who dropped out for any reason (OR 1.25, 95% CI 0.19 to 8.28; n = 201, studies = 6, very low-certainty evidence). When compared with midazolam, the evidence showed that ketamine increases remission rates at 24 hours (OR 2.21, 95% CI 0.67 to 7.32; n = 122,studies = 2, low-certainty evidence). The evidence is very uncertain about the response efficacy of ketamine at 24 hours in comparison with midazolam, and its ability to reduce depression rating scale scores at the same time point (OR 2.48, 95% CI 1.00 to 6.18; n = 296, studies = 4,very low-certainty evidence). There was no difference in the number of participants who dropped out of studies for any reason between ketamine and placebo (OR 0.33, 95% CI 0.05 to 2.09; n = 72, studies = 1, low-certainty evidence). Esketamine treatment likely results in a large increase in participants achieving remission at 24 hours compared with placebo (OR 2.74, 95% CI 1.71 to 4.40; n = 894, studies = 5, moderate-certainty evidence). Esketamine probably results in decreases in depression rating scale scores at 24 hours compared with placebo (SMD -0.31, 95% CI -0.45 to -0.17; n = 824, studies = 4, moderate-certainty evidence). Our findings show that esketamine increased response rates, although this evidence is uncertain (OR 2.11, 95% CI 1.20 to 3.68; n = 1071, studies = 5, low-certainty evidence). There was no evidence that participants assigned to esketamine treatment dropped out of trials more frequently than those assigned to placebo for any reason (OR 1.58, 95% CI 0.92 to 2.73; n = 773, studies = 4,moderate-certainty evidence). We found very little evidence for the remaining glutamate receptor modulators. We rated the risk of bias as low or unclear for most domains, though lack of detail regarding masking of treatment in the studies reduced our certainty in the effect for all outcomes.
Authors' conclusions: Our findings show that ketamine and esketamine may be more efficacious than placebo at 24 hours. How these findings translate into clinical practice, however, is not entirely clear. The evidence for use of the remaining glutamate receptor modulators is limited as very few trials were included in the meta-analyses for each comparison and the majority of comparisons included only one study. Long term non-inferiority RCTs comparing repeated ketamine and esketamine, and rigorous real-world monitoring are needed to establish comprehensive data on safety and efficacy.
Trial registration: ClinicalTrials.gov NCT02417064 NCT03039192 NCT00408031 NCT00977353 NCT03097133 NCT01304147 NCT00680433 NCT00610649 NCT02418585 NCT00491686 NCT00781742 NCT00344682 NCT01457677 NCT00986479 NCT03965871 NCT04019704 NCT04210804.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Rebecca Dean: none known.
Claudia Hurducas: none known.
Sarah Hollingsworth: none known.
Tahnee Marquardt: none known.
Stella Spyridi: none known.
Phil Cowen: PJC has a patent with the University of Oxford on the use of ebselen in treatment‐resistant depression
Keith Hawton: none known.
Rupert McShane: Rupert McShane runs NHS and self‐pay ketamine clinics for Oxford Health NHS Foundation Trust. Rupert has undertaken educational and scientific advisory board work for Janssen Pharmaceuticals to support educational and research activity, no funds are received personally. Janssen supported Rupert's attendance at the APA conference in New York in 2018. Rupert has undertaken scientific advisory board work for Sage pharmaceuticals, no funds are directly received.
Erick Turner: Erick Turner has no financial interest in esketamine, ketamine or competing treatments. Erick previously worked as a Medical Officer for the US Food and Drug Administration (FDA), charged with reviewing applications submitted by pharmaceutical companies and determining whether the evidence on drug efficacy and safety met the FDA’s criteria for US marketing approval. Erick is a former member of the Psychopharmacologic Drugs Advisory Committee which, together with the Drug Safety and Risk Management Advisory Committee, convened in Feb 2019, to advise the FDA on whether to approve esketamine, although Erick did not take part in that particular meeting due to matters related to the government shutdown.
Andrea Cipriani: Andrea Cipriani has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma.
Figures
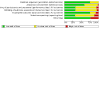




































































































































































































































































































































































































































































Update of
-
Ketamine and other glutamate receptor modulators for depression in adults.Cochrane Database Syst Rev. 2015 Sep 23;(9):CD011612. doi: 10.1002/14651858.CD011612.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Sep 12;9:CD011612. doi: 10.1002/14651858.CD011612.pub3. PMID: 26395901 Updated.
Comment in
-
Commentary on Cochrane review: "Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder".J Psychopharmacol. 2023 Aug;37(8):836-844. doi: 10.1177/02698811221123046. Epub 2022 Oct 11. J Psychopharmacol. 2023. PMID: 36218274 Free PMC article.
References
References to studies included in this review
Abbasinazari 2015 {published data only}
Amidfar 2016 {published data only}
-
- Amidfar M, Khiabany M, Kohi A, Salardini E, Arbabi M, Roohi Azizi M, et al. Effect of memantine combination therapy on symptoms in patients with moderate-to-severe depressive disorder: ramdomized, double-blind, placebo-controlled study. Journal of Clinical Pharmacy and Therapeutics 2016;42(1):44-50. - PubMed
Anderson 2017 {published data only}
-
- Anderson IM, Blamire A, Branton T, Clark R, Downey D, Dunn G, et al. Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine-ECT): a multicentre, double-blind, randomised, parallel-group, superiority trial. Lancet Psychiatry 2017;4(5):365-77. - PMC - PubMed
Arabzadeh 2018 {published data only}
-
- Arabzadeh S, Hakkikazazi E, Shahmansouri N, Tafakhori A, Ghajar A, Jafarinia M, et al. Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double-blind controlled trial. Journal of Affective Disorders 2018;235:236-41. - PubMed
Berk 2014 {published and unpublished data}
-
- Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, et al. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. Journal of Clinical Psychiatry 2014;75:628-36. - PubMed
Berman 2000 {published and unpublished data}
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 2000;47:351-4. - PubMed
Canuso 2018 {published and unpublished data}
-
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind randomised placebo-controlled study. American Journal of Psychiatry 2018;175(7):620630. [DOI: 10.1176/appi.ajp.2018.17060720] - DOI - PubMed
Carspecken 2018 {published data only}
-
- Carspecken CW, Borisovskaya A, Lan S-T, Heller K, Buchholz J, Ruskin D, et al. Ketamine anesthesia does not improve depression scores in electroconvulsive therapy: a randomised clinical trial. Journal of Neurosurgical Anesthesiology 2018;30(4):305-13. [DOI: 10.1097/ANA.0000000000000511] - DOI - PubMed
Chen 2017 {published data only}
-
- Chen Q, Min S, Hao X, Peng L, Meng H, Luo Q, et al. Effect of Low dose of ketamine on learning memory function in patients undergoing electroconvulsive therapy-a randomized, double-blind, controlled clinical study. Journal of ECT 2017;33(2):89-95. - PubMed
Chen 2018 {published data only}
-
- Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment resistant depression: a randomized control study. Journal of Affective Disorders 2018;225:709-14. [DOI: 10.1016/j.jad.2017.09.008] - DOI - PubMed
Correia‐Melo 2020 {published data only (unpublished sought but not used)}
-
- Correia-Melo FS, Leala GC, Vieira F, Jesus-Nunes AP, Mello RP, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double blind, non-inferiority study. Journal of Affective Disorders 29 July 2019;264:527-34. - PubMed
Daly 2018 {published data only}
Downey 2016 {published data only}
-
- Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. European Neuropsychopharmacology 2016;26(6):994-1003. [DOI: ] - PubMed
Fava 2018 {published and unpublished data}
-
- Fava M, Freeman PM, Flynn M, Judge H, Hoepnner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenousketamine as adjunctive therapy in treatment-resistant depression(TRD). Molecular Psychiatry 2020;25(7):1592-603. [DOI: 10.1038/s41380-018-0256-5] - DOI - PMC - PubMed
Fedgchin 2019 {published and unpublished data}
-
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane L, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressantin treatment-resistant depression: results of a randomized, double-blind, active-controlled study(TRANSFORM-1). International Journal of Neuropsychopharmacology 2019;22(10):616-30. [DOI: 10.1093/ijnp/pyz039] - DOI - PMC - PubMed
-
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Randomized, double-blind study of fixed-dose intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. In: Poster presentation at the 9th Biennial Conference of the International Society for Affective Disorders and the Houston Mood Disorders Conference. September 2018.
Fernie 2017 {published data only}
Fu 2020 {published and unpublished data}
-
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (Aspire I). Journal of Clinical Psychiatry 2020;81(3):e1-e9. [https://doi.org/10.4088/JCP.19m13191] - PubMed
Gálvez 2018 {published data only}
-
- Gálvez V, Huggins C, Glue P, Martin D, Somogyi AA, Alonzo A, et al. Repeated intranasal ketamine for treatment-resistant depression - the way to go? Results from a pilot randomised controlled trial. Journal of Psychopharmacology 2018;32(4):397-407. - PubMed
Ghasemi 2013 {published and unpublished data}
-
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Research 2013;215:355-61. - PubMed
Grunebaum 2018 {published data only}
Heresco‐Levy 2006 {published and unpublished data}
-
- Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. Journal of Affective Disorders 2006;93:239-43. - PubMed
Heresco‐Levy 2013 {published and unpublished data}
-
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A Randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. International Journal of Neuropsychopharmacology 2013;16:501-6. - PubMed
Hu 2016 {published data only}
-
- Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a ransomized, placebo-controlled 4-week study. Psychological Medicine 2016;46(3):623-35. - PubMed
Huang 2013 {published data only}
-
- Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biological Psychiatry 2013;74(10):734-41. - PubMed
Ibrahim 2012a {published and unpublished data}
-
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs Add-on riluzole: Results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 2012;37:1526-33. [Riluzole] - PMC - PubMed
Ibrahim 2012b {published and unpublished data}
-
- Ibrahim L, Diazgranados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring J, et al. A randomized, placebo-controlled crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. Journal of Clinical Psychopharmacology 2012;32:551-7. - PMC - PubMed
Ionescu 2018 {published data only}
-
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. Journal of Affective Disorders September 2018;243:516-24. [DOI: ] - PubMed
Ionescu 2020 {published and unpublished data}
-
- Ionescu DF, Fu D-J, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). International Journal of Neuropsychopharmacology 2020;24(1):1-10. [DOI: 10.1093/ijnp/pyaa068] - DOI - PMC - PubMed
Jagtiani 2014 {published data only}
-
- Jagtiani A, Khurana H, Malhotra N, Gandhi R. efficacy of ketamine versus thiopentone-assisted modified electroconvulsive therapy in major depression. Journal of ECT September 2014;30(3):251-6. [DOI: 10.1097/YCT.0000000000000158] - DOI
Jarventausta 2013 {published and unpublished data}
-
- Jarventausta K, Chrapek W, Kampman O, Tuohimaa K, Bjorkqvist M, Hakkinen H, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. Journal of ECT 2013;29:158-61. - PubMed
Kuşçu 2015 {published data only}
Lapidus 2014 {published and unpublished data}
Li 2016 {published data only}
Loo 2012 {published and unpublished data}
-
- Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. Journal of Affective Disorders 2012;142:233-40. - PubMed
Michelson 2007 {published and unpublished data}
-
- Michelson D, Adler LA, Amsterdam JD, Dunner DL, Nierenberg AA, Reimherr FW, et al. Addition of atomoxetine for depression incompletely responsive to sertraline: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2007;68:582-7. - PubMed
Murrough 2013 {published and unpublished data}
Nations 2012 (part I) {published and unpublished data}
-
- Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, Zraket D, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial. Journal of Psychopharmacology 2012;26:1525-39. - PubMed
Nations 2012 (part II) {published and unpublished data}
-
- Nations KR, Dogterom P, Bursi R, Schipper J, Greenwald S, Zraket D, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: An exploratory, randomized, double-blind, placebo-controlled trial. Journal of psychopharmacology 2012;26:1525-39. - PubMed
Ochs‐Ross 2020 {published data only}
-
- Ochs-Ross R, Daly EJ, Lane R, Zhang Y, Lim P, Foster K, et al. Efficacy and safety of intranasal esketamine plus an oral antidepressant in elderly patients with treatment-resistant depression. In: Poster presentation at the 2018 Annual Meeting of the American Psychiatric Association. May 2018.
-
- Ochs-Ross R, Daly EKJ, Zhang Y, Lane R, Lim P, Randall LM et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression - TRANSFORM-3. Am J of Geriatric Psychiatry 2020;28(2):121-141. [DOI: ] - PubMed
Omranifard 2014 {published and unpublished data}
Popova 2019 {published and unpublished data}
-
- Popova V, Daly EK, Madhukar T, Cooper K, Lane R, Lim P et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. American Journal of Psychiatry June 2019;176(6):428-38. - PubMed
Preskorn 2008 {published and unpublished data}
-
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. Journal of Clinical Psychopharmacology 2008;28:631-7. - PubMed
Preskorn 2015 {published data only}
-
- Preskorn S, Macaluso M, Mehra V, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-Methyl-D-Aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. Journal of Psychiatric Practice March 2015;21(2):140-149. [DOI: 10.1097/01.pra.0000462606.17725.93] - DOI - PubMed
Quiroz 2016 {published data only}
Roohi‐Azizi 2017 {published data only}
-
- Roohi-Azizi M, Arabzadeh S, Amidfar M, Salimi S, Zarindast MR, Ralaei A, et al. Citicoline combination therapy for major depressive disorder: A randomized, double-blind, placebo-controlled trial. Clinical Neuropharmacology January/February 2017;40(1):5. [DOI: 10.1097/WNF.0000000000000185] - DOI - PubMed
Salardini 2016 {published data only}
-
- Salardini E, Zeinoddini A, Mohammadinejad P, Khodaie-Ardakani M-R, Zahraei N, Zeinoddini A, et al. Riluzole combination therapy for moderate-to-severe major depressive disorder: A randomized, double-blind, placebo-controlled trial. Journal of Psychiatric Research 2016;75:24-30. [DOI: 10.1016/j.jpsychires.2016.01.003] - DOI - PubMed
Salehi 2015 {published data only}
-
- Salehi B, Mohammadbeigi A, Kamali AR, Taheri-Nejad MR, Moshiri I. Impact comparison of ketamine and sodium thiopental on anesthesia during electroconvulsive therapy in major depression patients with drug‑resistant; a double‑blind randomized clinical trial. Annals of Cardiac Anaesthesia 2015;18:486-90. [DOI: 10.4103/0971-9784.166444] - DOI - PMC - PubMed
Sanacora 2014 (a) {published and unpublished data}
Sanacora 2014 (b) {published and unpublished data}
Sanacora 2017 {published data only}
-
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, et al. Adjunctive lanicemine (AZD6765) in oatients with major depressive disorder and history of inadequate response to antidepressants: randomized, placebo-controlled study. Neuropsychopharmacology 2017;42:844853. [DOI: 10.1038/npp.2016.224] - DOI - PMC - PubMed
Shams Alizadeh 2015 {published data only}
-
- Shams Alizadeh NS, Maroufi A, Nasseri K, Sadeghi Najafabadi SH, Mousavi Taghiabad A, Gharibi F, et al. Antidepressant effect of combined ketamine and electroconvulsive therapy on patients with major depressive disorder: a randomized trial. Iranian Journal of Psychiatry and Behavioural Sciences 2015;9(3):e1578. - PMC - PubMed
Shiroma 2020 {published and unpublished data}
-
- Shiroma PR, Thuas P, Wels J, Albott S, Erbes C, Tye S, et al. A randomized, double-blind, active placebo-controlled study of efficacy, safety and durability of repeated vs single subanesthetic ketamine for treatment-resistant depression. Translational Psychiatry 2020;10(1):206. [DOI: ] - PMC - PubMed
Singh 2016 a {published data only}
-
- Singh JB, Fedgchin M, Dally EJ, De Boer P, Cooper K, Lim P, et al. A Double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. American Journal of Psychiatry August 2016;173(8):816-26. [DOI: 10.1176/appi.ajp.2016.16010037] - DOI - PubMed
Singh 2016 b {published data only}
-
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L. Intravenous esketamine in adult treatment-resistant depression: A double-blind, double-randomization, placebo-controlled study. Biological Psychiatry September 2016;80:424-431. [DOI: 10.1016/j.biopsych.2015.10.018] - DOI - PubMed
Smith 2013 {published and unpublished data}
-
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry Diseases of the Nervous System 2013;74:966-73. - PMC - PubMed
Sos 2013 {published and unpublished data}
-
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinology Letters 2013;34(4):287-93. - PubMed
Su 2017 {published data only}
-
- Chen MH, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, et al. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. Journal of Affected Disorders 2019;259:15-20. [CLINICAL TRIAL REGISTRATION: R000019001- UMIN000016985] [DOI: ] - PubMed
Sumner 2020 {published and unpublished data}
-
- Sumner RL, McMillian R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, Deng C, Hay J, Ponton R, Kirk IJ, Sundram F, Muthukumaraswamy SD. Ketamine Enhances Visual Sensory Evoked Potential Long-term Potentiation in Patients With Major Depressive Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2020;5(1):45-55. [DOI: 10.1016/j.bpsc.2019.07.002] - DOI - PubMed
Tiger 2020 {published and unpublished data}
Umbricht 2020 {published data only (unpublished sought but not used)}
-
- Umbricht D, Niggli M, Sanwald-Ducray P, Deptula D, Moore R, Grunbauer W, et al. Randomised, double-blind, placebo-controlled trial of the mglu2/3 negative allosteric modulator decoglurant in partially refractory major depressive disorder. Journal of Clinical Psychiatry 2020;81(4):18m12470. [DOI: 10.4088/JCP.18m12470] - DOI - PubMed
Yoosefi 2014 {published and unpublished data}
-
- Yoosefi A, Sepehri AS, Kargar M, Akhondzadeh S, Sadeghi M, Rafei A, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: a randomized, double-blind study. Journal of ECT 2014;30:15-21. - PubMed
Zarate 2006a {published and unpublished data}
-
- Zarate C, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry 2006;63:856-64. - PubMed
Zarate 2006b {published and unpublished data}
-
- Zarate C, Singh JB, Quiroz JA, De JG, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. American Journal of Psychiatry 2006;163:153-5. - PubMed
References to studies excluded from this review
Aftanas 2019 {published data only}
-
- Aftanas LI, Bazanova OM, Khabarov AN, Pustovoit SM, Brack IV. Placebo-controlled study of xenon effect on the emotions and frequency of the EEG alpha-oscillations [Russian]. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk 2019;74(5):342-50.
Barzman 2005 {published data only}
-
- Barzman DH, DelBello MP, Kowatch RA, Gernert B, Fleck DE, Pathak S, et al. The effectiveness and tolerability of aripiprazole for pediatric bipolar disorders: A retrospective chart review. Journal of Child and Adolescent Psychopharmacology 2005;14:593-600. - PubMed
Bondolfi 2000 {published data only}
-
- Bondolfi G, Lissner C, Kosel M, Eap CB, Baumann P. Fluoxetine augmentation in citalopram non-responders: Pharmacokinetic and clinical consequences. International Journal of Neuropsychopharmacology 2000;3:55-60. - PubMed
Burger 2016 {published data only}
-
- Burger J, Capobianco M, Lovern R, Boche B, Ross E, Darracq MA, et al. A double-blinded, randomized, placebo-controlled sub-dissociative dose ketamine pilot study in the treatment of acute depression and suicidality in a military emergency department setting. Military Medicine 2016;181(10):1195-9. - PubMed
Chen 2020 {published data only}
-
- Chen MH, Lin WC, Wu HJ, Bai YM, Li CT, Tsai SJ, et al. Efficacy of low-dose ketamine infusion in anxious vs nonanxious depression: revisiting the adjunctive ketamine study of Taiwanese patients with treatment-resistant depression. CNS Spectrums 2020;26(4):1-6. [DOI: 10.1017/S1092852920001194] - DOI - PubMed
Erdil 2015 {published data only}
-
- Erdil F, Begeç Z, Kayhan GE, Yoloğlu S, Ersoy MÖ, Durmuş M. Effects of sevoflurane or ketamine on the QTc interval during electroconvulsive therapy. Journal of Anasthesia 2015;29(2):180-5. - PubMed
Giese 2014 {published data only}
-
- Giese M, Beck J, Brand S, Muheim F, Hemmeter U, Hatzinger M, et al. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. Journal of Psychiatric Research 2014;59:1-7. - PubMed
Huey 2005 {published data only}
-
- Huey ED, Dustin IH, Overman GP, Mirza N, Sunderland T. Development of subtle psychotic symptoms with memantine: A case report 4 [letter]. Journal of Clinical Psychiatry 2005;66:658-9. - PubMed
Irwin 2010 {published data only}
Liebrenz 2009 {published data only}
-
- Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World Journal of Biological Psychiatry 2009;10:640-3. - PubMed
O'Gorman 2019 {published data only}
-
- O'Gorman C, Anderson A, Jacobson M, Jones A, Tabuteau H. P. 601 AXS-05 (dextromethorphan/bupropion), a novel, oral, investigational agent for major depressive disorder: Results of a randomized, double-blind, active-controlled, multi-center trial (ASCEND).. European Neuropsychopharmacology 2019;29:S410.
Park 2020 {published data only}
Rasmussen 2014 {published data only}
-
- Rasmussen KG, Kung S, Lapid MI, Oesterle TS, Geske JR, Nuttall GA, et al. A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Research 2014;215:362-5. - PubMed
Rosenblat 2019 {published data only}
-
- Rosenblat, JD. Potential differences in antidepressant effects of oral ketamine liquid suspension versus compounded capsules. British Journal of Psychiatry 2019;215(1):434. - PubMed
Sharma 2020 {published data only}
-
- Sharma, R K. Antidepressant effects of ketamine and ECT: a pilot comparison. Journal of Affective Disorders 2020;276:260-6. - PubMed
Shiroma 2020 {published data only}
Zhang 2018 {published data only}
-
- Zhang M, Rosenheck R, Lin X, Li Q, Zhou Y, Xiao, et al. A randomized clinical trial of adjunctive ketamine anesthesia in electro-convulsive therapy for depression. Journal of Affective Disorders 2018;227:372-8. - PubMed
Zhong 2016 {published data only}
-
- Zhong X, He H, Zhang C, Wang Z, Jiang M, Li Q, et al. Mood and neuropsychological effects of different doses of ketamine in electroconvulsive therapy for treatment-resistant depression. Journal of Affective Disorders 2016;201:124-30. - PubMed
References to studies awaiting assessment
IRCT201104092266N2 {published data only}
-
- IRCT201104092266N2. Comparison of the effect of electroconvulsive therapy and intravenous infusion of ketamine on control and relapse of depressive symptoms in depressive cases who are candidates of electro convulsive therapy. http://www.irct.ir/searchresult.php?id=2266&number=2 (August 28th 2015).
ISRCTN87057460 {published data only}
-
- ISRCTN87057460. Effectiveness of the dual serotonin norepinephrine reuptake inhibitor venlafaxine in depressed patients. http://www.isrctn.com/ISRCTN87057460 (August 28th 2015).
NCT01046630 {published data only}
-
- NCT01046630. A Phase I, multi-centre, double-blind, placebo-controlled parallel group study to assess the pharmacoMRI effects of AZD6765 in male and female subjects fulfilling the criteria for major depressive disorder. http://clinicaltrials.gov/show/NCT01046630 (August 28th 2015).
NCT01482221 {published data only}
-
- NCT01482221. A multicenter, randomized, double-blind, parallel group, placebo-controlled, phase IIb efficacy and safety study of adjunctive AZD6765 in patients with major depressive disorder (MDD) and a history of inadequate response to antidepressants study D6702C00031. http://clinicaltrials.gov/show/NCT01482221 (August 28th 2015).
NCT01627782 {published data only}
-
- NCT01627782. A double-blind, randomized, placebo-controlled, parallel group, dose frequency study of ketamine in subjects with treatment-resistant depression. http://clinicaltrials.gov/show/NCT01627782 (August 28th 2015).
References to ongoing studies
EUCTR2011‐001520‐37‐SE {published data only}
-
- EUCTR2011-001520-37-SE. Ketamine as an alternative to electroconvulsive therapy for treatment of major depressive disorder. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Vestibular... (August 28th 2015).
EUCTR2011‐005476‐41‐GB {published data only}
-
- EUCTR2011-005476-41-GB. Ketamine augmentation of ECT to improve outcomes in depression - Ketamine-ECT study. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-005476-41/GB (August 28th 2015). - PMC - PubMed
IRCT201307181556N54 {published data only}
-
- IRCT201307181556N54. Riluzole as adjuvant therapy in the treatment of moderate to severe major depression: a double - blind placebo-controlled trial. http://www.irct.ir/searchresult.php?keyword=&id=1556&number=54&a... (August 28th 2015).
NCT00988663 {published data only}
-
- NCT00988663. Memantine augmentation of electroconvulsive therapy in patients with major depression. http://clinicaltrials.gov/show/NCT00988663 (August 28th 2015).
NCT01179009 {published data only}
-
- NCT01179009. A safe ketamine-based therapy for treatment resistant depression. http://clinicaltrials.gov/show/NCT01179009 (August 28th 2015).
NCT01204918 {published data only}
-
- NCT01204918. Efficacy and tolerability of riluzole in treatment resistant depression. http://clinicaltrials.gov/show/NCT01204918 (August 28th 2015).
NCT01260649 {published data only}
-
- NCT01260649. N-methyl-D-aspartate antagonist (ketamine) augmentation of electroconvulsive treatment for severe major depression. http://clinicaltrials.gov/show/NCT01260649 (August 28th 2015).
NCT01441505 {published data only}
-
- NCT01441505. A study of ketamine as an antidepressant. http://clinicaltrials.gov/show/NCT01441505 (August 28th 2015).
NCT01557712 {published data only}
-
- NCT01557712. Estimate the efficiency of the association of an injection of ketamine and the venlafaxine in the severe major depressive disorder for six weeks (KETADEP). http://clinicaltrials.gov/show/NCT01557712 (August 28th 2015).
NCT01558063 {published data only}
-
- NCT01558063. The antidepressant action of ketamine: brain chemistry. http://clinicaltrials.gov/show/NCT01558063 (August 28th 2015).
NCT01613820 {published data only}
-
- NCT01613820. Combination of anticholinergic and glutamatergic effects in treatment-resistant major depressive disorder. A pilot study. http://clinicaltrials.gov/show/NCT01613820 (August 28th 2015).
NCT01667926 {published data only}
-
- NCT01667926. Randomized, double-blind ketamine augmentation in chronically suicidal, treatment-resistant major depression. http://clinicaltrials.gov/show/NCT01667926 (August 28th 2015).
NCT01684163 {published data only}
-
- NCT01684163. Phase 2, double-blind, placebo controlled, randomized withdrawal, parallel efficacy and safety study of GLYX-13 in subjects with inadequate/partial response to antidepressants during the current episode of major depressive disorder. http://clinicaltrials.gov/show/NCT01684163 (August 28th 2015).
NCT01700829 {published data only}
-
- NCT01700829. Ketamine vs. midazolam: testing rapid relief of suicide risk in depression. http://clinicaltrials.gov/show/NCT01700829 (August 28th 2015).
NCT01703039 {published data only}
-
- NCT01703039. Riluzole augmentation pilot in depression (RAPID) trial. http://clinicaltrials.gov/show/NCT01703039 (August 28th 2015).
NCT01868802 {published data only}
-
- NCT01868802. Ketamine for treatment-resistant depression: a multicentric clinical trial in Mexican population. http://clinicaltrials.gov/show/NCT01868802 (August 28th 2015).
NCT01880593 {published data only}
-
- NCT01880593. Ketamine plus lithium as a novel pharmacotherapeutic strategy in treatment-resistant depression. http://clinicaltrials.gov/show/NCT01880593 (August 28th 2015).
NCT01881763 {published data only}
-
- NCT01881763. Comparing therapeutic efficacy and cognitive side effects of electroconvulsive therapy (ECT) using ketamine versus methohexital anesthesia. http://clinicaltrials.gov/show/NCT01881763 (August 28th 2015).
NCT01882829 {published data only}
-
- NCT01882829. Targeting the NMDA glutamate receptor as novel antidepressant strategy: a pilot clinical trial of nuedexta in treatment-resistant major depression. http://clinicaltrials.gov/show/NCT01882829 (August 28th 2015).
NCT01887990 {published data only}
-
- NCT01887990. Treatment of suicidal ideation with intravenous ketamine infusion. http://clinicaltrials.gov/show/NCT01887990 (August 28th 2015).
NCT01902004 {published data only}
-
- NCT01902004. Treatment of geriatric depression with mild cognitive impairment: a double-blind placebo-controlled trial of namenda (memantine) augmentation of lexapro (escitalopram) in depressed patients at least 60 years of age. http://clinicaltrials.gov/show/NCT01902004 (August 28th 2015).
NCT01920555 {published data only}
-
- NCT01920555. Double-blind, placebo-controlled trial of ketamine therapy in treatment-resistant depression (TRD). http://clinicaltrials.gov/show/NCT01920555 (August 28th 2015).
NCT01935115 {published data only}
-
- NCT01935115. A prospective randomized double blinded control trial using ketamine or propofol anesthesia for electroconvulsive therapy: improving treatment-resistant depression. http://clinicaltrials.gov/show/NCT01935115 (August 28th 2015).
NCT01944293 {published data only}
-
- NCT01944293. Ketamine vs. midazolam in bipolar depression. http://clinicaltrials.gov/show/NCT01944293 (August 28th 2015).
NCT01945047 {published data only}
-
- NCT01945047. Phase 2 optimization of the antidepressant action of ketamine in treatment-resistant depression and investigations on its mechanism of action. http://clinicaltrials.gov/show/NCT01945047 (August 28th 2015).
NCT01957410 {published data only}
-
- NCT01957410. An open-label and double-blind study to investigate evoked potentials as markers of ketamine-induced cortical plasticity in subjects with major depressive disorder. http://clinicaltrials.gov/show/NCT01957410 (August 28th 2015).
NCT02012335 {published data only}
-
- NCT02012335. Ketamine use in electroconvulsive therapy: clinical, cognitives and neurotrophic outcomes. http://clinicaltrials.gov/show/NCT02012335 (August 28th 2015).
NCT02014363 {published data only}
-
- NCT02014363. Double blind, non-inferiority study to evaluate the antidepressant activity of ETS6103 compared with amitriptyline in the treatment of major depressive disorder (MDD) in patients who have an unsatisfactory response to selective serotonin re-uptake Inhibitors (SSRIs). http://clinicaltrials.gov/show/NCT02014363 (August 28th 2015).
NCT02037503 {published data only}
-
- NCT02037503. Effect of oral ketamine treatment on suicidal ideation and drug resistant major depression, a clinical and fMRI study. http://clinicaltrials.gov/show/NCT02037503 (August 28th 2015).
NCT02067793 {published data only}
-
- NCT02067793. Phase 2, randomized, double-blind, multiple-dose level, placebo controlled, single intravenous dose, parallel efficacy and safety study of NRX-1074 in subjects with major depressive disorder. http://clinicaltrials.gov/show/NCT02067793 (August 28th 2015).
NCT02106325 {published data only}
-
- NCT02106325. A randomized, double-blinded controlled trial of an N-methyl D-aspartate antagonist as a rapidly-acting antidepressant in depressed emergency department patients. http://clinicaltrials.gov/show/NCT02106325 (August 28th 2015).
NCT02139540 {published data only}
-
- NCT02139540. Nitrous oxide as treatment for major depression - a pilot study. http://clinicaltrials.gov/show/NCT02139540 (August 28th 2015).
NCT02153502 {published data only}
-
- NCT02153502. A phase 2, multicenter, randomized, double-blind, placebo-controlled study to assess the efficacy, safety, and tolerability of AVP-786 (deuterium modified dextromethorphan hydrobromide/quinidine sulfate) as an adjunctive therapy in patients with Major depressive disorder with an inadequate response to antidepressant treatment. http://clinicaltrials.gov/show/NCT02153502 (August 28th 2015).
NCT02267980 {published data only}
-
- NCT02267980. Effect of the addition of ketamine to sevoflurane anesthesia in electroconvulsive therapy. http://clinicaltrials.gov/show/NCT02267980 (August 28th 2015). - PubMed
NCT02295787 {published data only}
-
- NCT02295787. Intranasal ketamine for late-life depression and suicidal ideation. http://clinicaltrials.gov/show/NCT02295787 (August 28th 2015).
NCT02299440 {published data only}
-
- NCT02299440. Evaluation of the effects of ketamine in the acute phase of suicidal ideation: a multicenter randomized double-blind trial. http://clinicaltrials.gov/show/NCT02299440 (August 28th 2015).
NCT02305394 {published data only}
-
- NCT02305394. Effect of subanesthetic dose of ketamine combined with propofol on cognitive function in depressive patients undergoing electroconvulsive therapy - a randomized control double-blind clinical trial. http://clinicaltrials.gov/show/NCT02305394 (August 28th 2015).
NCT04082858 {published data only}
-
- NCT04082858. Ketamine interleaved with electroconvulsive therapy for depression. https://clinicaltrials.gov/show/NCT04082858. 2019.
NCT04116528 {published data only}
-
- Opiate Suicide Study in Patients With Major Depression (AFSP). Ongoing study. August 1, 2020. Contact author for more information.
NCT04234776 {published data only}
-
- NCT04234776. Intramuscular ketamine versus aripiprazole and escitalopram in the treatment of resistant depression. https://clinicaltrials.gov/show/NCT04234776. 2019.
NCT04399070 {published data only}
-
- NCT04399070. The Effect of S-ketamine for patients undergoing electroconvulsive therapy (ECT. https://clinicaltrials.gov/show/NCT04399070. 2020.
NTR3753 {published data only}
-
- NTR3753. ECT and Memantine. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3753 (August 28th 2015).
Additional references
Abdallah 2018
-
- Abdallah C, Averil, LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, et al. Rapamycin, an Immunosuppressant and mTORC1 Inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double-blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv 2018:p.500959.
Aitken 2019
Altman 1996
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. Washington, D.C: American Psychiatric Association, 1980.
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edition - Revised. Washington, D.C: American Psychiatric Association, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, D.C: American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association, 2000.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington (VA): American Psychiatric Association, 2013.
Arroll 2009
Atkins 2004
Bandelow 2006
-
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? Journal of Clinical Psychiatry 2006;67(9):1428–34. - PubMed
Birkenhager 1995
-
- Birkenhager TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. International Clinical Psychopharmacology 1995;10(3):181-95. - PubMed
Brookes 2001
-
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomized controlled trials: quantifying the risks of false-positives and false-negatives. Health Technology Assessment 2001;5(33):1–56. - PubMed
Brookes 2004
-
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. Journal of Clinical Epidemiology 2004;57(3):229-36. - PubMed
Brown 2019
Caddy 2014
Cipriani 2009
-
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009;373(9665):746–58. - PubMed
Cipriani 2010
Cipriani 2012
Cipriani 2019
Cipriani 2020
-
- Cipriani A, Ioannidis JP, Rothwell PM, Glasziou P, Li T, Hernandez AF, et al. Generating comparative evidence on new drugs and devices after approval. Lancet 2020;395(10228):998-1010. [DOI: ] - PubMed
Coppen 1967
-
- Coppen A. The biochemistry of affective disorders. British Journal of Psychiatry 1967;113:1237-64. - PubMed
Cotter 2001
-
- Cotter D, Mackay D, Landau S, Kerwin R Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder.. Archives of General Psychiatry 2001;58(6):545553. - PubMed
Coyle 2015
-
- Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Human Psychopharmacology 2015;30:152-63. - PubMed
Dean 2021
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses.. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 6.2 edition. Cochrane, 2021.
De Leo 2014
-
- De Leo D, Too LS. Suicide and depression. In: Okpaku, SO, editors(s). Essentials of Global Mental Health. Cambridge: Cambridge University Press, 2014:367-73.
Diamond 2014
-
- Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. Journal of Psychopharmacology 2014;28(6):536-44. - PubMed
Donohue 2007
-
- Donohue JM, Pincus HA. Reducing the societal burden of depression: a review of economic costs, quality of care and effects of treatment. Pharmacoeconomics 2007;25:7-24. - PubMed
Dozois 2004
-
- Dozois D JA, Dobson KS. Depression. In: Antony M M, Barlow D H, editors(s). Handbook of Assessment and Treatment Planning for Psychological Disorders. New York: Guilford Press, 2004:259-99.
Efthimiou 2019
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology 2002;31(1):140–9. - PubMed
Feighner 1972
-
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26(1):57–63. - PubMed
Furukawa 2002
-
- Furukawa TA, Guyatt GH, Griffith LE. An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002;31(1):72–6. - PubMed
Furukawa 2005
-
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analysis. International Clinical Psychopharmacology 2005;20(1):49–52. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7–10. - PubMed
Furukawa 2007a
-
- Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta-analyses. JAMA 2007;297(5):468–70. - PubMed
Furukawa 2007b
-
- Furukawa TA, Akechi T, Azuma H, Okuyama T, Higuchi T. Evidence-based guidelines for interpretation of the Hamilton Rating Scale for Depression. Journal of Clinical Psychopharmacology 2007;27(5):531–4. - PubMed
Godfrey 2018
-
- odfrey KM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. Journal of Psychiatric Research. 2018;105:33-44. - PubMed
Godlewska 2018
Goh 2019
GradePro GDT 2020
-
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Available from gradepro.org. 2020.
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): U.S. Department of Health and Human Services, 1976.
Guyatt 1998
Hamilton 1960
Hasler 2010a
Hawton 2009
-
- Hawton K, Heeringen K. Suicide. Lancet 2009;373:1372-81. - PubMed
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].
Higgins 2011d
-
- Higgins JP, Green S (editors). 7.7.3.8 Combining groups. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 edition. Cochrane Collaboration, March 2011.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editors). Cochrane Handbook for Systematic Reviews of Interventions. Available from www.training.cochrane.org/handbook. 2020;Version 6.1.
Hirschfeld 2000
-
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. Jourmal of Clinical Psychiatry 2000;61 Suppl 6:4-6. - PubMed
Jones 2019
Koesters 2013
-
- Koesters M, Guaiana G, Cipriani A, Becker T, Barbui C. Agomelatine efficacy and acceptability revisited: systematic review and meta-analysis of published and unpublished randomised trials. British Journal of Psychiatry 2013;203(3):179-87. - PubMed
Li 2010
Lim 2018
Magni 2013
Malhi 2019
Marcantoni 2020
-
- Marcantoni W, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. Journal of Affective Disorders 2020;277:831-41. [DOI: 10.1016/j.jad.2020.09.007] - DOI - PubMed
Mavridis 2014
-
- Mavridis D, Chaimani A, Efthimiou O, Leucht S Salanti G. Addressing missing outcome data in meta-analysis. Evidence Based Mental Health 2014;17(3):85-9. - PubMed
McCloud 2015
McGirr 2015
-
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological Medicine 2015;45:693-704. - PubMed
Memon 2020
Moher 2009
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382–9. - PubMed
Moriguchi 2019
Muller 2003
-
- Muller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255–60. - PubMed
Naci 2020
-
- Naci H, Salcher-Konrad M, Kesselheim AS, Wieseler B, Rochaiz L, Redberg RF, et al. Generating comparative evidence on new drugs and devices before approval. Lancet 2020;395(10228):986-97. [DOI: ] - PubMed
Naughton 2014
-
- Naughton M, Clarke G, O'Leary OF, Cryan JF, Dinan TG. A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. Journal of Affective Disorders 2014;156:24-35. - PubMed
NICE 2009
-
- National Institute for Health and Clinical Excellence. Depression in adults: The treatment and management of depression in adults (CG90). http://publications.nice.org.uk/depression-in-adults-cg90 2009.
Petty 1984
-
- Petty F, Sherman AD. Plasma GABA levels in psychiatric illness. Journal of Affective Disorders 1984;6:131-8. - PubMed
Price 2014
Rapaport 2005
-
- Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. American Journal of Psychiatry 2005;162:1171-8. - PubMed
Revman 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager. Version Version 5.4. The Cochrane Collaboration, 2020.
Ruhe 2007
-
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular Psychiatry 2007;12:331-59. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations.. The GRADE Working Group, 2013.
Solmi 2016
-
- Solmi M, Veronese N, Zaninotto L, Loos ML, Gao K, Schaffer A, et al. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: a comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectrums 2016;21(5):403-18. [DOI: 10.1017/S1092852916000523] - DOI - PubMed
Spitzer 1978
-
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives General Psychiatry 1978;35(6):773–82. - PubMed
Sterne 2000
-
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119–29. - PubMed
Suh 1997
-
- Suh T, Gallo JJ. Symptom profiles of depression among general medical service users compared with specialty mental health service users. Psychological Medicine 1997;27(5):1051–63. - PubMed
Turner 2019
Valentine 2011
Watanabe 2008
-
- Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, et al. Mirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: systematic review and meta-analysis. Journal of Clinical Psychiatry 2008;69(9):1404-15. - PubMed
Watanabe 2011
WHO 1992
-
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders. Geneva: WHO, 1992.
WHO 2008a
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). New York, NY: WHO, 2008.
WHO 2008b
-
- World Health Organization (WHO). The global burden of disease: 2004 update. Geneva: WHO, 2008.
WHO 2012
-
- World Health Organization (WHO). In: Sixty-fifth World Health Assembly: daily notes on proceedings. Geneva: World Health Organization, 2012.
Wilkinson 2019
Williams 2018
Yoon 2019
Zheng 2019
-
- Zheng W, Cai DB, Zheng W, Sim K, Ungvari GS, Peng XJ, et al. Brexanolone for postpartum depression: a meta-analysis of randomized controlled studies.. Psychiatry Research 2019;279:83-9. - PubMed
Zheng 2020
Zhou 2020
-
- Zhou X, Teng T, Zhang Y, Del Giovane C, Furukawa TA, Weisz JR, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry 2020;7(7):581-601. - PMC - PubMed
Zohar 2014
-
- Zohar J, Nutt DJ, Kupfer DJ, Moller HJ, Yamawaki S, Spedding M, et al. A proposal for an updated neuropsychopharmacological nomenclature. European Neuropsychopharmacology 2014;24(7):1005-14. - PubMed
References to other published versions of this review
Amit 2015
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

