Insect eggs trigger systemic acquired resistance against a fungal and an oomycete pathogen
- PMID: 34510462
- PMCID: PMC9292583
- DOI: 10.1111/nph.17732
Insect eggs trigger systemic acquired resistance against a fungal and an oomycete pathogen
Abstract
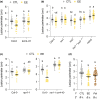
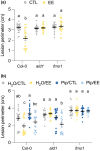
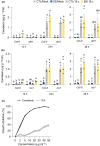
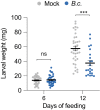
Plants are able to detect insect eggs deposited on leaves. In Arabidopsis, eggs of the butterfly species Pieris brassicae (common name large white) induce plant defenses and activate the salicylic acid (SA) pathway. We previously discovered that oviposition triggers a systemic acquired resistance (SAR) against the bacterial hemibiotroph pathogen Pseudomonas syringae. Here, we show that insect eggs or treatment with egg extract (EE) induce SAR against the fungal necrotroph Botrytis cinerea BMM and the oomycete pathogen Hyaloperonospora arabidopsidis Noco2. This response is abolished in ics1, ald1 and fmo1, indicating that the SA pathway and the N-hydroxypipecolic acid (NHP) pathway are involved. Establishment of EE-induced SAR in distal leaves potentially involves tryptophan-derived metabolites, including camalexin. Indeed, SAR is abolished in the biosynthesis mutants cyp79B2 cyp79B3, cyp71a12 cyp71a13 and pad3-1, and camalexin is toxic to B. cinerea in vitro. This study reveals an interesting mechanism by which lepidopteran eggs interfere with plant-pathogen interactions.
Keywords: Botrytis cinerea; Pieris brassicae; herbivore interactions; indolic metabolism; insect eggs; plant; systemic acquired resistance (SAR).
© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Figures


Similar articles
-
Pieris brassicae eggs trigger interplant systemic acquired resistance against a foliar pathogen in Arabidopsis.New Phytol. 2020 Dec;228(5):1652-1661. doi: 10.1111/nph.16788. Epub 2020 Aug 2. New Phytol. 2020. PMID: 32619278
-
Insect eggs induce a systemic acquired resistance in Arabidopsis.Plant J. 2014 Dec;80(6):1085-94. doi: 10.1111/tpj.12707. Plant J. 2014. PMID: 25329965
-
Priming of camalexin accumulation in induced systemic resistance by beneficial bacteria against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000.J Exp Bot. 2022 Jun 2;73(11):3743-3757. doi: 10.1093/jxb/erac070. J Exp Bot. 2022. PMID: 35191984
-
N-hydroxypipecolic acid and salicylic acid: a metabolic duo for systemic acquired resistance.Curr Opin Plant Biol. 2019 Aug;50:44-57. doi: 10.1016/j.pbi.2019.02.006. Epub 2019 Mar 27. Curr Opin Plant Biol. 2019. PMID: 30927665 Review.
-
Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity.Mol Plant. 2020 Jan 6;13(1):31-41. doi: 10.1016/j.molp.2019.12.008. Epub 2019 Dec 18. Mol Plant. 2020. PMID: 31863850 Review.
Cited by
-
Priming of Arabidopsis resistance to herbivory by insect egg deposition depends on the plant's developmental stage.J Exp Bot. 2022 Aug 11;73(14):4996-5015. doi: 10.1093/jxb/erac199. J Exp Bot. 2022. PMID: 35522985 Free PMC article.
-
Plant defensive responses to insect eggs are inducible by general egg-associated elicitors.Sci Rep. 2024 Jan 11;14(1):1076. doi: 10.1038/s41598-024-51565-y. Sci Rep. 2024. PMID: 38212511 Free PMC article.
-
The Key Role of Plant Hormone Signaling Transduction and Flavonoid Biosynthesis Pathways in the Response of Chinese Pine (Pinus tabuliformis) to Feeding Stimulation by Pine Caterpillar (Dendrolimus tabulaeformis).Int J Mol Sci. 2024 Jun 8;25(12):6354. doi: 10.3390/ijms25126354. Int J Mol Sci. 2024. PMID: 38928063 Free PMC article.
-
Insect egg-induced innate immunity: Who benefits?PLoS Pathog. 2023 Jan 19;19(1):e1011072. doi: 10.1371/journal.ppat.1011072. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36656837 Free PMC article.
-
Metabolites and Plant Hormones Related to the Resistance Response to Feeding Stimulation and Leaf Clipping Control in Chinese Pine (Pinus tabuliformis Carr.).Curr Issues Mol Biol. 2023 Jan 30;45(2):1086-1099. doi: 10.3390/cimb45020072. Curr Issues Mol Biol. 2023. PMID: 36826017 Free PMC article.
References
-
- Albert I, Böhm H, Albert M, Feiler CE, Imkampe J, Wallmeroth N, Brancato C, Raaymakers TM, Oome S, Zhang H et al. 2015. An RLP23–SOBIR1–BAK1 complex mediates NLP‐triggered immunity. Nature Plants 1: 15140. - PubMed
-
- Bednarek P. 2012. Chemical warfare or modulators of defence responses – the function of secondary metabolites in plant immunity. Current Opinion in Plant Biology 15: 407–414. - PubMed
-
- Bednarek P, Piślewska‐Bednarek M, Loren V, van Themaat E, Maddula RK, Svatos A, Schulze‐Lefert P. 2011. Conservation and clade‐specific diversification of pathogen‐inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytologist 192: 713–726. - PubMed
-
- Bednarek P, Pislewska‐Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez‐Vallet A et al. 2009. A glucosinolate metabolism pathway in living plant cells mediates broad‐spectrum antifungal defense. Science 323: 101–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

