Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety
- PMID: 34511939
- PMCID: PMC8418359
- DOI: 10.2147/IDR.S315727
Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety
Abstract
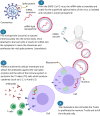
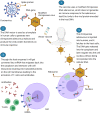
This review takes into consideration the principal vaccines developed against the SARS-CoV-2 in this unprecedented period of Covid-19 pandemic. We evaluated the mechanism of action of each vaccine as well as the efficacy, the safety and the storage temperature. In addition, the problem of the dose units, the vaccinal strategy, the activity of alternative compounds such as the monoclonal antibodies and especially the issue of the virus variants were also described in detail. Four vaccines are currently used in Italy: Pfizer-BioNTech mRNA BNT162b2 (Comirnaty) (USA), Moderna mRNA 1273 (USA), Astra-Zeneca ChAdOx1-S (recombinant) viral vector adenovirus belonging to Oxford (UK) and Pomezia (Italy), Janssen (two recombinant viral vector adenoviruses) belonging to Johnson & Johnson (USA). The efficacy of Pfizer and Moderna for preventing disease or severe disease results 95-87.5% and 94.5-100%, respectively. The efficacy of Astra-Zeneca and Janssen is about 70% and 65%, respectively; in the case of Janssen, it depends on the geographical area ranging from 72% to 57%. The problem of the administrated doses (one dose, two doses from the same vaccine or from different vaccines, half dose) is also discussed. The vaccination strategy based on the age group remains the simplest, most transparent and fair criterion. This strategy is also based on accelerating the administration of the vaccines, so that as many subjects as possible can be vaccinated quickly for achieving the "herd immunity". The monoclonal antibodies appeared to be a valid solution for the treatment of Covid-19 disease. Two antibodies (bamlanivimab and etesevimab) have just been approved by the FDA. They could also be used for the infection by virus variants which represent a big problem due to their higher transmissibility and virulence and to their lower response to the vaccines.
Keywords: Covid-19 vaccines; dose units; monoclonal antibodies; vaccinal platforms; vaccinal strategy; virus variants.
© 2021 Mascellino et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
"Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety" [Response To Letter].Infect Drug Resist. 2021 Oct 28;14:4501-4502. doi: 10.2147/IDR.S344230. eCollection 2021. Infect Drug Resist. 2021. PMID: 34737590 Free PMC article. No abstract available.
References
-
- Kumar S, Nyodu R, Maurya VK, Saxena SK.Morphology, genome organization, replication, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). coronavirus disease 2019 (COVID-19). Coronavirus Dis. 2020:23–31. doi:10.1007/978-981-15-4814-7_3 - DOI
-
- Ho D, Tsuji M. Infectious diseases: breakthroughs in developing vaccines, immune protective monitoring and measuring toxicity with functional proteomics. Isoplexis e-Book. 2020;3. Available from: https://isoplexis.com/literature/infectious-disease-breakthroughs-in-dev....
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous