Effect of Electro-Acupuncture at ST36 and SP6 on the cAMP -CREB Pathway and mRNA Expression Profile in the Brainstem of Morphine Tolerant Mice
- PMID: 34512242
- PMCID: PMC8431970
- DOI: 10.3389/fnins.2021.698967
Effect of Electro-Acupuncture at ST36 and SP6 on the cAMP -CREB Pathway and mRNA Expression Profile in the Brainstem of Morphine Tolerant Mice
Abstract
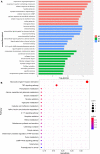
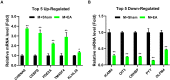
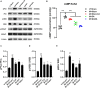
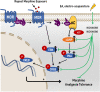
Undoubtedly, opioid drugs have been the most popular treatment for refractory pain since found, such as morphine. However, tolerance to the analgesic effects caused by repeated use is inevitable, which greatly limits the clinical application of these drugs. Nowadays, it has become the focus of the world that further development of non-opioid-based treatment along with efficient strategies to circumvent opioid tolerance are urgently needed clinically. Fortunately, electro-acupuncture (EA) provides an alternative to pharmaceutic treatment, remaining its potential mechanisms unclear although. This study was aimed to observe the effects of EA on morphine-induced tolerance in mice and discover its underlying mechanism. Tail-flick assay and hot-plate test were conducted to assess the development of tolerance to morphine-induced analgesia effect. As a result of repeated administration scheme (10 mg/kg, twice per day, for 7 days), approximately a two-fold increase was observed in the effective dose of 50% (ED50) of morphine-induced antinociceptive effect. Interestingly, by EA treatment (2/100Hz, 0.5, 1.0, and 1.5 mA, 30 min/day for 7 days) at the acupoints Zusanli (ST36) and Sanyinjiao (SP6), morphine ED50 curves was remarkably leftward shifted on day 8. In addition, the RNA sequencing strategy was used to reveal the potential mechanisms. Due to the well described relevance of cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), extracellular regulated protein kinases (ERK), and cAMP response element-binding (CREB) in brainstem (BS) to analgesia tolerance, the cAMP-PKA/ERK-CREB signaling was deeply concerned in this study. Based upon Enzyme-Linked Immunosorbent Assay, the up-regulation of the cAMP level was observed, whereas reversed with EA treatment. Similarly, western blot revealed the phosphorylation levels of PKA, ERK, and CREB were up-regulated in morphine tolerant mice, whereas the EA group showed a significantly reduced expression level instead. This study observed an attenuating effect of the EA at ST36 and SP6 on morphine tolerance in mice, and suggested several potential biological targets by RNA-seq, which include the cAMP-PKA/ERK-CREB signaling pathway, strongly supporting a useful treatment for combatting the opioid epidemic, and opioid-tolerant patients.
Keywords: CREB; PKA/ERK; cAMP; electro-acupuncture; morphine-induced analgesic tolerance.
Copyright © 2021 Wang, Qin, Wang, Yang, Liu, Li, Jiang, Lu, Wang and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- DeWire S. M., Yamashita D. S., Rominger D. H., Liu G., Cowan C. L., Graczyk T. M., et al. (2013). A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 344 708–717. 10.1124/jpet.112.201616 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

