Tomato Divinyl Ether-Biosynthesis Pathway Is Implicated in Modulating of Root-Knot Nematode Meloidogyne javanica's Parasitic Ability
- PMID: 34512679
- PMCID: PMC8424051
- DOI: 10.3389/fpls.2021.670772
Tomato Divinyl Ether-Biosynthesis Pathway Is Implicated in Modulating of Root-Knot Nematode Meloidogyne javanica's Parasitic Ability
Abstract
The role of the 9-lipoxygenase (9-LOX)-derived oxylipins in plant defense is mainly known in solanaceous plants. In this work, we identify the functional role of the tomato divinyl ether synthase (LeDES) branch, which exclusively converts 9-hydroperoxides to the 9-divinyl ethers (DVEs) colneleic acid (CA) and colnelenic acid (CnA), during infection by the root-knot nematode Meloidogyne javanica. Analysis of LeDES expression in roots indicated a concurrent response to nematode infection, demonstrating a sharp increase in expression during the molting of third/fourth-stage juveniles, 15 days after inoculation. Spatiotemporal expression analysis using an LeDES promoter:GUS tomato line showed high GUS activity associated with the developing gall; however the GUS signal became more constricted as infection progressed to the mature nematode feeding sites, and eventually disappeared. Wounding did not activate the LeDES promoter, but auxins and methyl salicylate triggered LeDES expression, indicating a hormone-mediated function of DVEs. Heterologous expression of LeDES in Arabidopsis thaliana rendered the plants more resistant to nematode infection and resulted in a significant reduction in third/fourth-stage juveniles and adult females as compared to a vector control and the wild type. To further evaluate the nematotoxic activity of the DVEs CA and CnA, recombinant yeast that catalyzes the formation of CA and CnA from 9-hydroperoxides was generated. Transgenic yeast accumulating CnA was tested for its impact on M. javanica juveniles, indicating a decrease in second-stage juvenile motility. Taken together, our results suggest an important role for LeDES as a determinant in the defense response during M. javanica parasitism, and indicate two functional modes: directly via DVE motility inhibition effect and through signal molecule-mediated defense reactions to nematodes that depend on methyl salicylate.
Keywords: Meloidogyne javanica; divinyl ether synthase; hormone signaling; innate immunity; oxylipins; plant defense signaling.
Copyright © 2021 Sanadhya, Kumar, Bucki, Fitoussi, Carmeli-Weissberg, Borenstein and Brown-Miyara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






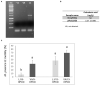
References
-
- Alkharouf N. W., Klink V. P., Chouikha I. B., Beard H. S., MacDonald M. H., Meyer S., et al. . (2006). Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224, 838–852. 10.1007/s00425-006-0270-8 - DOI - PubMed
-
- Bar-Or C., Kapulnik Y., Koltai H. (2005). A broad characterization of the transcriptional profile of the compatible tomato response to the plant parasitic root knot nematode Meloidogyne javanica. Eur. J. Plant Pathol. 111, 181–192. 10.1007/s10658-004-2134-z - DOI
-
- Cabrera J., Díaz-Manzano F. E., Sanchez M., Rosso M. N., Melillo T., Goh T., et al. . (2014). A role for LATERAL ORGAN BOUNDARIES-DOMAIN 16 during the interaction Arabidopsis-Meloidogyne spp. provides a molecular link between lateral root and root-knot nematode feeding site development. New Phytol. 203, 632–645. 10.1111/nph.12826 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

