Daucus carota DcPSY2 and DcLCYB1 as Tools for Carotenoid Metabolic Engineering to Improve the Nutritional Value of Fruits
- PMID: 34512681
- PMCID: PMC8427143
- DOI: 10.3389/fpls.2021.677553
Daucus carota DcPSY2 and DcLCYB1 as Tools for Carotenoid Metabolic Engineering to Improve the Nutritional Value of Fruits
Abstract
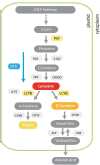
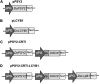
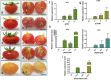
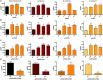
Carotenoids are pigments with important nutritional value in the human diet. As antioxidant molecules, they act as scavengers of free radicals enhancing immunity and preventing cancer and cardiovascular diseases. Moreover, α-carotene and β-carotene, the main carotenoids of carrots (Daucus carota) are precursors of vitamin A, whose deficiency in the diet can trigger night blindness and macular degeneration. With the aim of increasing the carotenoid content in fruit flesh, three key genes of the carotenoid pathway, phytoene synthase (DcPSY2) and lycopene cyclase (DcLCYB1) from carrots, and carotene desaturase (XdCrtI) from the yeast Xanthophyllomyces dendrorhous, were optimized for expression in apple and cloned under the Solanum chilense (tomatillo) polygalacturonase (PG) fruit specific promoter. A biotechnological platform was generated and functionally tested by subcellular localization, and single, double and triple combinations were both stably transformed in tomatoes (Solanum lycopersicum var. Microtom) and transiently transformed in Fuji apple fruit flesh (Malus domestica). We demonstrated the functionality of the S. chilense PG promoter by directing the expression of the transgenes specifically to fruits. Transgenic tomato fruits expressing DcPSY2, DcLCYB1, and DcPSY2-XdCRTI, produced 1.34, 2.0, and 1.99-fold more total carotenoids than wild-type fruits, respectively. Furthermore, transgenic tomatoes expressing DcLCYB1, DcPSY2-XdCRTI, and DcPSY2-XdCRTI-DcLCYB1 exhibited an increment in β-carotene levels of 2.5, 3.0, and 2.57-fold in comparison with wild-type fruits, respectively. Additionally, Fuji apple flesh agroinfiltrated with DcPSY2 and DcLCYB1 constructs showed a significant increase of 2.75 and 3.11-fold in total carotenoids and 5.11 and 5.84-fold in β-carotene, respectively whereas the expression of DcPSY2-XdCRTI and DcPSY2-XdCRTI-DcLCYB1 generated lower, but significant changes in the carotenoid profile of infiltrated apple flesh. The results in apple demonstrate that DcPSY2 and DcLCYB1 are suitable biotechnological genes to increase the carotenoid content in fruits of species with reduced amounts of these pigments.
Keywords: Daucus carota; DcLCYB1; DcPSY2; Malus domestica; Solanum lycopersicum; carotenoid; metabolic engineering; transgenic tomatoes.
Copyright © 2021 Arias, Arenas-M, Flores-Ortiz, Peirano, Handford and Stange.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

