Genome-wide activation screens to increase adeno-associated virus production
- PMID: 34513296
- PMCID: PMC8413672
- DOI: 10.1016/j.omtn.2021.06.026
Genome-wide activation screens to increase adeno-associated virus production
Abstract
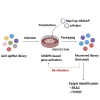
We describe a genome-wide screening strategy to identify target genes whose modulation increases the capacity of a cell to produce recombinant adeno-associated viral (AAV) vector. Specifically, a single-guide RNA (sgRNA) library for a CRISPR-based genome-wide transcriptional activation screen was inserted into an AAV vector, and iterative rounds of viral infection and rescue in HEK293 producer cells enabled the enrichment of sgRNAs targeting genes whose upregulation increased AAV production. Numerous gain-of-function targets were identified, including spindle and kinetochore associated complex subunit 2 (SKA2) and inositol 1, 4, 5-trisphosphate receptor interacting protein (ITPRIP). Furthermore, individual or combinatorial modulation of these targets in stable producer cell lines increased vector genomic replication and loading into AAV virions, resulting in up to a 3.8-fold increase in AAV manufacturing capacity. Our study offers an efficient approach to engineer viral vector producer cell lines and enhances our understanding of the roles of SKA2 and ITPRIP in AAV packaging.
Keywords: adeno-associated virus; cell engineering; gene therapy; high-throughput screening; vector manufacturing.
© 2021 The Authors.
Conflict of interest statement
C.R.B., D.S.O., and D.V.S. are inventors on patents related to cell lines for increased production of AAV. D.V.S. is a co-founder of 4D Molecular Therapeutics.
Figures




References
-
- O. of the Commissioner (2019). FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-innovat....
-
- O. of the Commissioner (2017). Press Announcements - FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. FDA. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm589467.htm.
-
- Spark Therapeutics LUXTURNA package insert. 1–16.
-
- AveXis (2019). ZOLGENSMA® (onasemnogene abeparvovec-xioi) suspension for intravenous infusion [package insert]. Bannockburn (IL): AveXis Inc; 2019 May. Available from: https://www.fda.gov/media/126109/download. 1–14.
-
- Gene Transfer Clinical Trial to Deliver rAAVrh74.MCK.GALGT2 for Duchenne Muscular Dystrophy ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03333590. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

