Dengue Virus Dysregulates Master Transcription Factors and PI3K/AKT/mTOR Signaling Pathway in Megakaryocytes
- PMID: 34513730
- PMCID: PMC8427595
- DOI: 10.3389/fcimb.2021.715208
Dengue Virus Dysregulates Master Transcription Factors and PI3K/AKT/mTOR Signaling Pathway in Megakaryocytes
Abstract
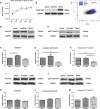
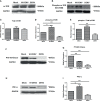
Dengue virus (DENV) infection can cause either self-limited dengue fever or hemorrhagic complications. Low platelet count is one of the manifestations of dengue fever. Megakaryocytes are the sole producers of platelets. However, the role of both host and viral factors in megakaryocyte development, maturation, and platelet production is largely unknown in DENV infection. PI3K/AKT/mTOR pathway plays a significant role in cell survival, maturation, and megakaryocyte development. We were interested to check whether pathogenic insult can impact this pathway. We observed decreased expression of most of the major key molecules associated with the PI3K/AKT/mTOR pathway in DENV infected MEG-01 cells. In this study, the involvement of PI3K/AKT/mTOR pathway in megakaryocyte development and maturation was confirmed with the use of specific inhibitors in infected MEG-01 cells. Our results showed that direct pharmacologic inhibition of this pathway greatly impacted megakaryopoiesis associated molecule CD61 and some essential transcription factors (GATA-1, GATA-2, and NF-E2). Additionally, we observed apoptosis in megakaryocytes due to DENV infection. Our results may suggest that DENV impairs PI3K/AKT/mTOR axis and molecules involved in the development and maturation of megakaryocytes. It is imperative to investigate the role of these molecules in the context of megakaryopoiesis during DENV infection to better understand the pathways and mechanisms, which in turn might provide insights into the development of antiviral strategies.
Keywords: AKT; GATA-2; MEG-01; NF-E2; dengue virus; mTOR; thrombopoietin.
Copyright © 2021 Lahon, Arya and Banerjea.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Basu A., Jain P., Gangodkar S. V., Shetty S., Ghosh K. (2008). Dengue 2 Virus Inhibits In Vitro Megakaryocytic Colony Formation and Induces Apoptosis in Thrombopoietin-Inducible Megakaryocytic Differentiation From Cord Blood CD34+ Cells. FEMS Immunol. Med. Microbiol. 53 (1), 46–51. 10.1111/j.1574-695X.2008.00399.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

