Extending traditional antibody therapies: Novel discoveries in immunotherapy and clinical applications
- PMID: 34514097
- PMCID: PMC8416972
- DOI: 10.1016/j.omto.2021.08.005
Extending traditional antibody therapies: Novel discoveries in immunotherapy and clinical applications
Abstract
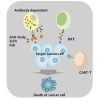
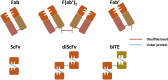
Immunotherapy has been well regarded as one of the safer and antigen-specific anti-cancer treatments compared to first-generation chemotherapy. Since Coley's discovery, researchers focused on engineering novel antibody-based therapies. Including artificial and modified antibodies, such as antibody fragments, antibody-drug conjugates, and synthetic mimetics, the variety of immunotherapy has been rapidly expanding in the last few decades. Genetic and chemical modifications to monoclonal antibody have been brought into academia, in vivo trials, and clinical applications. Here, we have looked around antibodies overall. First, we elucidate the antibody structure and its cytotoxicity mechanisms. Second, types of therapeutic antibodies are presented. Additionally, there is a summarized list of US Food and Drug Administration (FDA)-approved therapeutic antibodies and recent clinical trials. This review provides a comprehensive overview of both the general function of therapeutic antibodies and a few main variations in development, including recent advent with the proposed mechanism of actions, and we introduce types of therapeutic antibodies, clinical trials, and approved commercial immunotherapeutic drugs.
Keywords: T lymphocytes; anti-cancer therapy; apoptosis; cytotoxicity; immune-related adverse effects; immunotherapy; therapeutic antibody.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Oiseth S.J., Aziz M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017;3:250–261.
Publication types
LinkOut - more resources
Full Text Sources