Optical-Property-Enhancing Novel Near-Infrared Active Niosome Nanoformulation for Deep-Tissue Bioimaging
- PMID: 34514233
- PMCID: PMC8427633
- DOI: 10.1021/acsomega.1c02632
Optical-Property-Enhancing Novel Near-Infrared Active Niosome Nanoformulation for Deep-Tissue Bioimaging
Abstract
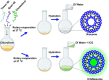
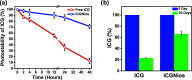
Indocyanine green (ICG) is a clinically approved near-infrared (NIR) contrast agent used in medical diagnosis. However, ICG has not been used to its fullest for biomedical imaging applications due to its low fluorescence quantum yield, aqueous instability, concentration-dependent aggregation, and photo and thermal degradations, leading to quenching of its fluorescence emission. In the present study, a nanosized niosomal formulation, ICGNiosomes (ICGNios), is fabricated to encapsulate and protect ICG from degradation. Interestingly, compared to free ICG, the ICGNios exhibited higher fluorescence quantum yield and fluorescence emission with a bathochromic shift. Also, ICGNios nanoparticles are biocompatible, biodegradable, and readily uptaken by the cells. Furthermore, ICGNios show more enhanced fluorescence intensity through ∼1 cm thick chicken breast tissue compared to free ICG, which showed minimal emission through the same thickness of tissue. Our results suggest that ICGNios could offer a promising platform for deep-tissue NIR in vivo imaging to visualize inaccessible tissue microstructures for disease diagnosis and therapeutics.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Zhao Y.; Van Rooy I.; Hak S.; Fay F.; Tang J.; Davies C. D. L.; Skobe M.; Fisher E. A.; Radu A.; Fayad Z. A.; De Mello Donegá C.; Meijerink A.; Mulder W. J. M. Near-Infrared Fluorescence Energy Transfer Imaging of Nanoparticle Accumulation and Dissociation Kinetics in Tumor-Bearing Mice. ACS Nano 2013, 7, 10362–10370. 10.1021/nn404782p. - DOI - PMC - PubMed
-
- Yoneya S.; Saito T.; Komatsu Y.; Koyama I.; Takahashi K.; Duvoll-Young J. Binding Properties of Indocyanine Green in Human Blood. Invest. Ophthalmol. Visual Sci. 1998, 39, 1286–1290. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

