Tolerability of Coronavirus Disease 2019 Vaccines, BNT162b2 and mRNA-1273, in Patients With Thymic Epithelial Tumors
- PMID: 34514444
- PMCID: PMC8423742
- DOI: 10.1016/j.jtocrr.2021.100229
Tolerability of Coronavirus Disease 2019 Vaccines, BNT162b2 and mRNA-1273, in Patients With Thymic Epithelial Tumors
Abstract
Introduction: Defects in immunologic self-tolerance result in an increased risk for development of paraneoplastic autoimmune diseases (ADs) and immune-mediated toxicity in response to immune stimulation in individuals with thymic epithelial tumors (TETs). We conducted a survey to evaluate the tolerability of coronavirus disease 2019 (COVID-19) mRNA vaccines in patients with TETs, including individuals with preexisting AD.
Methods: After reviewing published data on adverse events associated with the BNT162b2 (Pfizer, Inc., and BioNTech) and mRNA-1273 (ModernaTX, Inc.) mRNA vaccines, we designed and administered a questionnaire to participants at the following three time points: after each dose of vaccination and 1 month after the final dose. Questions related to AD and use of immunosuppressive drugs were included. Descriptive statistics were used to analyze data, and results were compared with previously described results related to the BNT162b2 and mRNA-1273 vaccines.
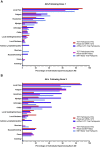
Results: From February 26 to June 1, 2021, we administered the survey to 54 participants (median age = 58 y, thymoma = 33, preexisting AD = 19). Common adverse events included injection site pain, fatigue, and headaches. There were no vaccination-related hospitalizations or deaths. Autoimmune flares occurred in three patients (16%) after the first dose and three patients (17%) after the second dose. Most AD flares were mild and self-limited. One patient (2%) was diagnosed with having a new AD after vaccination.
Conclusions: Tolerability of COVID-19 mRNA vaccines in patients with TETs is comparable to the general population. Most patients with preexisting AD did not experience disease flares, and the development of new AD was rare. Patients with TETs should be encouraged to get vaccinated against COVID-19 owing to the documented benefits of vaccination and manageable risk profile.
Keywords: Autoimmunity; COVID-19; Thymic carcinoma; Thymoma; Vaccination.
Figures
References
-
- Conforti F., Pala L., Giaccone G., De Pas T. Thymic epithelial tumors: from biology to treatment. Cancer Treat Rev. 2020;86:102014. - PubMed
-
- Marx A., Wilcox N., Leite M.I. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43:413–427. - PubMed
-
- Lippner E.A., Lewis D.B., Robinson W.H., Katsumoto T.R. Paraneoplastic and therapy-related immune complications in thymic malignancies. Curr Treat Options Oncol. 2019;20:62. - PubMed
LinkOut - more resources
Full Text Sources

