Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study
- PMID: 34514454
- PMCID: PMC8418937
- DOI: 10.1016/j.lanepe.2021.100208
Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study
Abstract
Background: SARS-CoV-2 mRNA vaccines have proven high efficacy, however, limited data exists on the duration of immune responses and their relation to age and side effects.
Methods: We studied the antibody and memory T cell responses after the two-dose BNT162b2 vaccine in 122 volunteers up to 6 months and correlated the findings with age and side effects.
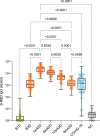
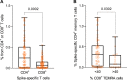
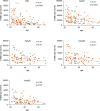
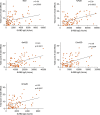
Findings: We found a robust antibody response to Spike protein after the second dose. However, the antibody levels declined at 12 weeks and 6 months post-vaccination, indicating a waning of the immune response over time. At 6 months after the second dose, the Spike antibody levels were similar to the levels in persons vaccinated with one dose or in COVID-19 convalescent individuals. The antibodies efficiently blocked ACE2 receptor binding to SARS-CoV-2 Spike protein of five variants of concern at one week but this was decreased at three months. 87% of individuals developed Spike-specific memory T cell responses, which were lower in individuals with increased proportions of immunosenescent CD8+ TEMRA cells. We found antibody response to correlate negatively with age and positively with the total score of vaccination side effects.
Interpretation: The mRNA vaccine induces a strong antibody response to SARS-CoV-2 and five VOCs at 1 week post-vaccination that decreases thereafter. T cell responses, although detectable in the majority, were lower in individuals with higher T cell immunosenescence. The deterioration of vaccine response suggests the need to monitor for the potential booster vaccination.
Keywords: SARS-CoV-2 mRNA vaccine; adverse effects; age; dynamics of the immune response.
© 2021 The Authors.
Conflict of interest statement
The authors have nothing to disclose.
Figures

References
-
- Shrotri M, Fragaszy E, Geismar C, et al. Spike-antibody responses following first and second doses of ChAdOx1 and BNT162b2 vaccines by age, gender, and clinical factors - a prospective community cohort study (Virus Watch) medRxiv. 2021 2021.05.12.21257102.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

