Use of Faecal Transplantation with a Novel Diet for Mild to Moderate Active Ulcerative Colitis: The CRAFT UC Randomised Controlled Trial
- PMID: 34514495
- PMCID: PMC8919830
- DOI: 10.1093/ecco-jcc/jjab165
Use of Faecal Transplantation with a Novel Diet for Mild to Moderate Active Ulcerative Colitis: The CRAFT UC Randomised Controlled Trial
Abstract
Background: We evaluated whether integration of novel diets for donors and patients, in addition to faecal transplantation [FT], could increase FT remission rate in refractory ulcerative colitis [UC].
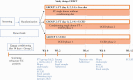
Methods: This was a blinded, randomised, controlled trial in adults with active UC, defined by a simple clinical colitis activity index [SCCAI] of ≥5 and ≤11 and endoscopic Mayo score 2-3, refractory to medication. Group 1 received free diet and single donor standard FT by colonoscopy on Day 1and rectal enemas on Days 2 and 14 without dietary conditioning of the donor. Group 2 received FT as above but with dietary pre-conditioning of the donor for 14 days and a UC Exclusion Diet [UCED] for the patients. Group 3 received the UCED alone. The primary endpoint was Week 8 clinical steroid-free remission, defined as SCCAI <3.
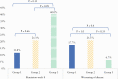
Results: Of 96 planned patients, 62 were enrolled. Remission Week 8 Group 1 was 2/17 [11.8%], Group 2 was 4/19 [21.1%], Group 3 was 6/15 [40%] [non-significant]. Endoscopic remission Group 1 was 2/17 [12%], Group 2 was 3/19 [16%], Group 3 was 4/15 [27%] [Group 1 vs 3 p = 0.38]. Mucosal healing [Mayo 0] was achieved only in Group 3 [3/15, 20%] vs 0/36 FT patients [p = 0.022]. Exacerbation of disease occurred in 3/17 [17.6%] of Group 1, 4/19 [21.1%] of Group 2, and 1/15 [6.7%] of Group 3 [Group 2 vs 3, p = 0.35].
Conclusions: UCED alone appeared to achieve higher clinical remission and mucosal healing than single donor FT with or without diet. The study was stopped for futility by a safety monitoring board.
Keywords: Ulcerative colitis; diet; faecal transplantation; fibre; microbiome.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures
Comment in
-
Eating for Two: Diet and the Microbiome in Ulcerative Colitis.J Crohns Colitis. 2022 Mar 14;16(3):341-342. doi: 10.1093/ecco-jcc/jjab181. J Crohns Colitis. 2022. PMID: 34718490 Free PMC article. No abstract available.