Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential
- PMID: 34514892
- PMCID: PMC8808084
- DOI: 10.1177/00220345211037247
Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential
Abstract
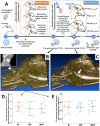
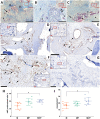
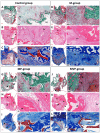
The clinical translation of bone tissue engineering for reconstructing large bone defects has not advanced without hurdles. The in vivo bioreactor (IVB) concept may therefore bridge between bone tissue engineering and reconstructive surgery by employing the patient body for prefabricating new prevascularized tissues. Ideally, IVB should minimize the need for exogenous growth factors/cells. Periosteal tissues are promising for IVB approaches to prefabricate tissue-engineered bone (TEB) flaps. However, the significance of preserving the periosteal vascular supply has not been adequately investigated. This study assessed muscle IVB with and without periosteal/pericranial grafts and flaps for prefabricating TEB flaps to reconstruct mandibular defects in sheep. The sheep (n = 14) were allocated into 4 groups: muscle IVB (M group; nM = 3), muscle + periosteal graft (MP group; nMP = 4), muscle + periosteal flap (MVP group; nMVP = 4), and control group (nControl = 3). In the first surgery, alloplastic bone blocks were implanted in the brachiocephalic muscle (M) with a periosteal graft (MP) or with a vascularized periosteal flap (MVP). After 9 wk, the prefabricated TEB flaps were transplanted to reconstruct a mandibular angle defect. In the control group, the defects were reconstructed by non-prevascularized bone blocks. Computed tomography (CT) scans were performed after 13 wk and after 23 wk at termination, followed by micro-CT (µCT) and histological analyses. Both CT and µCT analysis revealed enhanced new bone formation and decreased residual biomaterial volume in the MVP group compared with control and MP groups, while the M group showed less new bone formation and more residual biomaterial. The histological analysis showed that most of the newly formed bone emerged from defect edges, but larger areas of new bone islands were found in MP and MVP groups. The MVP group showed enhanced vascularization and higher biomaterial remodeling rates. The periosteal flaps boosted the reconstructive potential of the prefabricated TEB flaps. The regenerative potential of the periosteum was manifested after the transplantation into the mechanically stimulated bony defect microenvironment.
Keywords: bioreactors; flap prefabrication; mandibular reconstruction; periosteum; sheep; vascularization.
Conflict of interest statement
Figures


References
-
- Aaron JE, Skerry TM. 1994. Intramembranous trabecular generation in normal bone. Bone Miner. 25(3):211–230. - PubMed
-
- Ayoub A, Challa SR, Abu-Serriah M, McMahon J, Moos K, Creanor S, Odell E. 2007. Use of a composite pedicled muscle flap and rhBMP-7 for mandibular reconstruction. Int J Oral Maxillofac Surg. 36(12):1183–1192. - PubMed
-
- Battaglia S, Ratti S, Manzoli L, Marchetti C, Cercenelli L, Marcelli E, Tarsitano A, Ruggeri A. 2020. Augmented reality-assisted periosteum pedicled flap harvesting for head and neck reconstruction: an anatomical and clinical viability study of a galeo-pericranial flap. J Clin Med. 9(7):2211. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

