Improved Dialysis Removal of Protein-Bound Uraemic Toxins with a Combined Displacement and Adsorption Technique
- PMID: 34515053
- PMCID: PMC9227672
- DOI: 10.1159/000518065
Improved Dialysis Removal of Protein-Bound Uraemic Toxins with a Combined Displacement and Adsorption Technique
Abstract
Introduction: Protein-bound uraemic toxins (PBUTs) are poorly removed by conventional dialytic techniques, given their high plasma protein binding, and thus low, free (dialysable) plasma concentration. Here, we evaluated and compared PBUTs removal among conventional haemodialysis (HD), adsorption-based HD, displacement-based HD, and their 2 combinations both in vitro and in vivo.
Methods: The removal of PBUTs, including 3-carboxy-4-methyl-5-propyl-2-furan-propanoic acid (CMPF), p-cresyl sulphate (PCS), indoxyl sulphate (IS), indole-3-acetic acid (3-IAA), and hippuric acid, was first evaluated in an in vitro single-pass HD model. Adsorption consisted of adding 40 g/L bovine serum albumin (Alb) to the dialysate and displacement involved infusing fatty acid (FA) mixtures predialyser. Then, uraemic rats were treated with either conventional HD, Alb-based HD, lipid emulsion infusion-based HD or their combination to calculate the reduction ratio (RR), and the total solute removal (TSR) of solutes after 4 h of therapy.
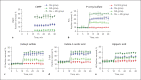
Results: In vitro dialysis revealed that FAs infusion prefilter increased the removal of PCS, IS, and 3-IAA 3.23-fold, 3.01-fold, and 2.24-fold, respectively, compared with baseline and increased the fractional removal of CMPF from undetectable at baseline to 14.33 ± 0.24%, with a dialysis efficacy markedly superior to Alb dialysis. In vivo dialysis showed that ω-6 soybean oil-based lipid emulsion administration resulted in higher RRs and more TSRs for PCS, IS, and 3-IAA after 4-h HD than the control, and the corresponding TSR values for PCS and IS were also significantly increased compared to that of Alb dialysis. Finally, the highest dialysis efficacy for highly bound solute removal was always observed with their combination both in vitro and in vivo.
Conclusions: The concept of combined displacement- and adsorption-based dialysis may open up new avenues and possibilities in the field of dialysis to further enhance PBUTs removal in end-stage renal disease.
Keywords: Adsorption technology; Displacer augmented dialysis technique; End-stage renal disease; Haemodialysis; Protein-bound uraemic toxins.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003 May;63((5)):1934–43. - PubMed
-
- Niwa T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013 May;35 Suppl 2:20–5. - PubMed
-
- Meijers BK, Bammens B, Verbeke K, Evenepoel P. A review of albumin binding in CKD. Am J Kidney Dis. 2008 May;51((5)):839–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

