Hydrogen Peroxide Is Crucial for NLRP3 Inflammasome-Mediated IL-1β Production and Cell Death in Pneumococcal Infections of Bronchial Epithelial Cells
- PMID: 34515145
- PMCID: PMC9149442
- DOI: 10.1159/000517855
Hydrogen Peroxide Is Crucial for NLRP3 Inflammasome-Mediated IL-1β Production and Cell Death in Pneumococcal Infections of Bronchial Epithelial Cells
Abstract
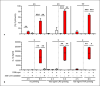
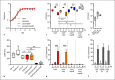
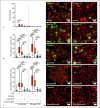
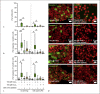
Epithelial cells play a crucial role in detection of the pathogens as well as in initiation of the host immune response. Streptococcus pneumoniae (pneumococcus) is a typical colonizer of the human nasopharynx, which can disseminate to the lower respiratory tract and subsequently cause severe invasive diseases such as pneumonia, sepsis, and meningitis. Hydrogen peroxide (H2O2) is produced by pneumococci as a product of the pyruvate oxidase SpxB. However, its role as a virulence determinant in pneumococcal infections of the lower respiratory tract is not well understood. In this study, we investigated the role of pneumococcal-derived H2O2 in initiating epithelial cell death by analyzing the interplay between 2 key cell death pathways, namely, apoptosis and pyroptosis. We demonstrate that H2O2 primes as well as activates the NLRP3 inflammasome and thereby mediates IL-1β production and release. Furthermore, we show that pneumococcal H2O2 causes cell death via the activation of both apoptotic as well as pyroptotic pathways which are mediated by the activation of caspase-3/7 and caspase-1, respectively. However, H2O2-mediated IL-1β release itself occurs mainly via apoptosis.
Keywords: Apoptosis; Caspase-1; Caspase-3/7; Cell death; IL-1β; NLRP3 inflammasome; Pyroptosis; Streptococcus pneumoniae.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
ALK-JNK signaling promotes NLRP3 inflammasome activation and pyroptosis via NEK7 during Streptococcus pneumoniae infection.Mol Immunol. 2023 May;157:78-90. doi: 10.1016/j.molimm.2023.03.016. Epub 2023 Mar 29. Mol Immunol. 2023. PMID: 37001294 Review.
-
Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia.Oncotarget. 2015 Dec 29;6(42):44161-78. doi: 10.18632/oncotarget.6592. Oncotarget. 2015. PMID: 26683708 Free PMC article.
-
The Role of NLRP3 Inflammasome in Pneumococcal Infections.Front Immunol. 2020 Dec 14;11:614801. doi: 10.3389/fimmu.2020.614801. eCollection 2020. Front Immunol. 2020. PMID: 33424869 Free PMC article. Review.
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
UFL1 modulates NLRP3 inflammasome activation and protects against pyroptosis in LPS-stimulated bovine mammary epithelial cells.Mol Immunol. 2019 Aug;112:1-9. doi: 10.1016/j.molimm.2019.04.023. Epub 2019 May 8. Mol Immunol. 2019. PMID: 31078114
Cited by
-
Canonical and non-canonical functions of NLRP3.J Adv Res. 2023 Nov;53:137-151. doi: 10.1016/j.jare.2023.01.001. Epub 2023 Jan 4. J Adv Res. 2023. PMID: 36610670 Free PMC article. Review.
-
Heme-Mediated Selection of Encapsulated Streptococcus pneumoniae in the Lungs by Oxidative Stress.bioRxiv [Preprint]. 2025 Jun 7:2023.11.14.567109. doi: 10.1101/2023.11.14.567109. bioRxiv. 2025. Update in: Emerg Microbes Infect. 2025 Dec;14(1):2532685. doi: 10.1080/22221751.2025.2532685. PMID: 38014009 Free PMC article. Updated. Preprint.
-
Mechanisms of Action of Ozone Therapy in Emerging Viral Diseases: Immunomodulatory Effects and Therapeutic Advantages With Reference to SARS-CoV-2.Front Microbiol. 2022 Apr 21;13:871645. doi: 10.3389/fmicb.2022.871645. eCollection 2022. Front Microbiol. 2022. PMID: 35531273 Free PMC article. Review.
-
Pyroptosis: a double-edged sword in lung cancer and other respiratory diseases.Cell Commun Signal. 2024 Jan 15;22(1):40. doi: 10.1186/s12964-023-01458-w. Cell Commun Signal. 2024. PMID: 38225586 Free PMC article. Review.
-
Streptococcus pneumoniae Impairs Maturation of Human Dendritic Cells and Consequent Activation of CD4+ T Cells via Pneumolysin.J Innate Immun. 2022;14(5):569-580. doi: 10.1159/000522339. Epub 2022 Mar 4. J Innate Immun. 2022. PMID: 35249041 Free PMC article.
References
-
- Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014 Oct 23;371((17)):1619–28. - PubMed
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008 Apr;6((4)):288–301. - PubMed
-
- Cuypers F, Klabunde B, Gesell Salazar M, Surabhi S, Skorka SB, Burchhardt G, et al. Adenosine triphosphate neutralizes pneumolysin-induced neutrophil activation. J Infect Dis. 2020 Oct 13;222((10)):1702–12. - PubMed
-
- Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idänpään-Heikkilä I, Rosenow C, et al. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996 Feb;19((4)):803–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

