Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study
- PMID: 34515521
- PMCID: PMC8817689
- DOI: 10.1089/dia.2021.0182
Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Study
Abstract
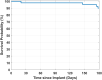
Background: Use of continuous glucose monitoring (CGM) systems is being rapidly adopted as standard of care for insulin-requiring patients with diabetes. The PROMISE study (NCT03808376) evaluated the accuracy and safety of the next-generation implantable Eversense CGM system for up to 180 days. Methods: This was a prospective multicenter study involving 181 subjects with diabetes at 8 USA sites. All subjects were inserted with a primary sensor. Ninety-six subjects had a second sensor, either an identical sensor or a modified sensor (sacrificial boronic acid [SBA]), inserted in their other arm (53 and 43 subjects, respectively). Accuracy was evaluated by comparing CGM to YSI 2300 glucose analyzer (Yellow Springs Instrument [YSI]) values during 10 clinic visits (day 1-180). Confirmed event detection rates, calibration stability, sensor survival, and serious adverse events (SAEs) were evaluated. Results: For primary sensors, the percent CGM readings within 20%/20% of YSI values was 92.9%; overall mean absolute relative difference (MARD) was 9.1%. The confirmed alert detection rate at 70 mg/dL was 93% and at 180 mg/dL was 99%. The median percentage of time for one calibration per day was 56%. Sixty-five percent of the primary sensors survived to 180 days. For the SBA sensors, the percent CGM readings within 20%/20% of YSI values was 93.9%; overall MARD was 8.5%. The confirmed alert detection rate at 70 mg/dL was 94% and at 180 mg/dL was 99%. The median percentage of time for one calibration per day was 63%. Ninety percent of the SBA sensors survived to 180 days. No device- or insertion/removal procedure-related SAEs were reported. Conclusion: These data show the next-generation Eversense CGM system had sustained accuracy and safety up to 180 days, with an improved calibration scheme and survival, using the primary or SBA sensors.
Keywords: Continuous glucose monitoring; Eversense; Implantable sensor; PROMISE study.
Conflict of interest statement
S.K.G. reports consultant fees from Medtronic, Novo Nordisk, Zealand, Lifescan, Roche, and Lilly; and research grants through the University of Colorado from Lilly, Novo Nordisk, Medtronic, Dexcom, T1D Exchange, Helmsley Trust, NIDDK, and JDRF. D.L. reports research grants from Medtronic, Dexcom, Abbott, AbbVie, Provention Bio, Sanvita, Senseonics, and TrialNet. B.B. reports consultant fees to his employer from Lexicon, Lilly, Medtronic, Novo Nordisk, Xeris, Zealand; research support from Abbott, BioProvention, Dexcom, Diasome, Janssen, JDRF, Lexicon, Lilly/BI, MannKind, Medtronic, NIH, Novo Nordisk, Pfizer, Sanofi, Senseonics, Viacyte, and Xeris; speaker's bureau from Astra Zeneca, BI, Lilly, MannKind, Medtronic, Novo Nordisk, andSanofi; and stock holdings in Aseko and Senseonics. M.P.C., on behalf of Diablo Clinical Research, reports research support from Abbott Diabetes Care, biolinq, Dexcom, Medtronic, SanVita, Senseonics, Abbott Point of Care, Ascensia, Novo Nordisk, Merck, Boehringer Ingelheim, and Helixmith. T.S.B. reports research support from Abbott Diabetes, Abbott Rapid Diagnostics, Biolinq, Capillary Biomedical, Dexcom, Eli Lilly, Kowa, Lexicon, Livongo, Medtronic, Novo Nordisk, REMD, Sanofi, Sanvita, Senseonics, Viacyte, vTv Therapeutics, and Zealand Pharma; and consulting and speaker honoraria from Abbott, Lifescan, Medtronic, Novo, Sanofi, BD, Medtronic, and Sanofi. R.L.B. has received research funding from Abbott, Ascensia, Medtronic, Novo, Lilly, Sanvita, Allergan, and Senseonics. D.S.D. reports no disclosures. A.R.C. reports no disclosures. H.A. received research grants through the University of Colorado from Senseonics, Dexcom, Eli Lilly, REMD Biotherapeutics, IM Therapeutics, Institute for the Advancement of Food and Nutrition Sciences, and Mannkind; and honorarium from the American Diabetes Association for speaking and consulting. A.D., K.S.T., and F.R.K. are employees and stockholders of Senseonics.
Figures
References
-
- American Diabetes Association. American Diabetes Association standards of medical care in diabetes 2021. Diabetes Care 2021;44(Suppl. 1):S85–S99. - PubMed
-
- Lind M, Polonsky W, Hirsch I, et al. : Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily injections—the GOLD Randomized Clinical Trial. JAMA 2017;317:379–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical