Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing
- PMID: 34515917
- PMCID: PMC8438099
- DOI: 10.1007/s40820-021-00717-0
Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing
Abstract
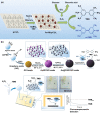
Nanomaterial-based artificial enzymes (or nanozymes) have attracted great attention in the past few years owing to their capability not only to mimic functionality but also to overcome the inherent drawbacks of the natural enzymes. Numerous advantages of nanozymes such as diverse enzyme-mimicking activities, low cost, high stability, robustness, unique surface chemistry, and ease of surface tunability and biocompatibility have allowed their integration in a wide range of biosensing applications. Several metal, metal oxide, metal-organic framework-based nanozymes have been exploited for the development of biosensing systems, which present the potential for point-of-care analysis. To highlight recent progress in the field, in this review, more than 260 research articles are discussed systematically with suitable recent examples, elucidating the role of nanozymes to reinforce, miniaturize, and improve the performance of point-of-care diagnostics addressing the ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to the end user) criteria formulated by World Health Organization. The review reveals that many biosensing strategies such as electrochemical, colorimetric, fluorescent, and immunological sensors required to achieve the ASSURED standards can be implemented by using enzyme-mimicking activities of nanomaterials as signal producing components. However, basic system functionality is still lacking. Since the enzyme-mimicking properties of the nanomaterials are dictated by their size, shape, composition, surface charge, surface chemistry as well as external parameters such as pH or temperature, these factors play a crucial role in the design and function of nanozyme-based point-of-care diagnostics. Therefore, it requires a deliberate exertion to integrate various parameters for truly ASSURED solutions to be realized. This review also discusses possible limitations and research gaps to provide readers a brief scenario of the emerging role of nanozymes in state-of-the-art POC diagnosis system development for futuristic biosensing applications.
Keywords: ASSURED diagnostics; Biosensing; Catalytic nanomaterials; Nanozymes; Point-of-care diagnosis.
© 2021. The Author(s).
Figures










References
-
- Organización Mundial de la Salud, The global burden of disease 2004. Updat. World Heal. Organ 146 (2004). ISBN 978 92 4 156371 0 https://apps.who.int/iris/bitstream/handle/10665/43942/9789241563710_eng...
-
- Gao X, Xu L-P, Zhou S-F, Liu G, Zhang X. Recent advances in nanoparticles-based lateral flow biosensors. Am. J. Biomed. Sci. 2014;6(1):41–57. doi: 10.5099/aj140100041. - DOI
-
- Sumner JB. Enzyme urease. J. Biol. Chem. 1926;69:435–441. doi: 10.4159/harvard.9780674366701.c115. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
