Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications
- PMID: 34515975
- PMCID: PMC8478757
- DOI: 10.1007/s12325-021-01882-9
Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications
Abstract
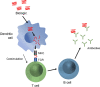
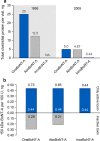
Botulinum neurotoxins (BoNTs) are proteins produced by bacteria of the Clostridium family. Upon oral ingestion, BoNT causes the neuroparalytic syndrome botulism. There are seven serotypes of BoNT (serotypes A-G); BoNT-A and BoNT-B are the botulinum toxin serotypes utilized for therapeutic applications. Treatment with BoNT injections is used to manage chronic medical conditions across multiple indications. As with other biologic drugs, immunogenicity after long-term treatment with BoNT formulations may occur, and repeated use can elicit antibody formation leading to clinical nonresponsiveness. Thus, approaching BoNT treatment of chronic conditions with therapeutic formulations that minimize stimulating the host immune response while balancing patient responsiveness to therapy is ideal. Immunogenicity is a clinical limitation in many settings that use biologic drugs for treatment, and clinically relevant immunogenicity reduction has been achieved through engineering smaller protein constructs and reducing unnecessary formulation components. A similar approach has influenced the evolution of BoNT formulations. Three BoNT-A products and one BoNT-B product have been approved by the Food and Drug Administration (FDA) for therapeutic use: onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, and rimabotulinumtoxinB; a fourth BoNT-A product, daxibotulinumtoxinA, is currently under regulatory review. Additionally, prabotulinumtoxinA is a BoNT-A product that has been approved for aesthetic indications but not therapeutic use. Here, we discuss the preclinical and clinical immunogenicity data that exist within the scientific literature and provide a perspective for considering immunogenicity as a key factor in choice of BoNT formulation.
Keywords: AbobotulinumtoxinA; Antibodies; Biologics; Clinical response; IncobotulinumtoxinA; Neutralizing; OnabotulinumtoxinA; Second generation.
© 2021. The Author(s).
Figures


References
-
- Frevert J, Dressler D. Clinical relevance of immunoresistance to botulinum therapy. In: Rosales RL, Dressler D, editors. Botulinum toxin therapy manual for dystonia and spasticity. IntechOpen; 2016. pp. 33–49.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

