Thalamic Influence on Slow Wave Slope Renormalization During Sleep
- PMID: 34516002
- PMCID: PMC9291607
- DOI: 10.1002/ana.26217
Thalamic Influence on Slow Wave Slope Renormalization During Sleep
Abstract
Objective: Slow waves are thought to mediate an overall reduction in synaptic strength during sleep. The specific contribution of the thalamus to this so-called synaptic renormalization is unknown. Thalamic stroke is associated with daytime sleepiness, along with changes to sleep electroencephalography and cognition, making it a unique "experiment of nature" to assess the relationship between sleep rhythms, synaptic renormalization, and daytime functions.
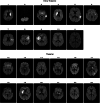
Methods: Sleep was studied by polysomnography and high-density electroencephalography over 17 nights in patients with thalamic (n = 12) and 15 nights in patients with extrathalamic (n = 11) stroke. Sleep electroencephalographic overnight slow wave slope changes and their relationship with subjective daytime sleepiness, cognition, and other functional tests were assessed.
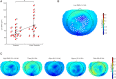
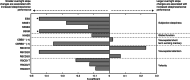
Results: Thalamic and extrathalamic patients did not differ in terms of age, sleep duration, or apnea-hypopnea index. Conversely, overnight slope changes were reduced in a large cluster of electrodes in thalamic compared to extrathalamic stroke patients. This reduction was related to increased daytime sleepiness. No significant differences were found in other functional tests between the 2 groups.
Interpretation: In patients with thalamic stroke, a reduction in overnight slow wave slope change and increased daytime sleepiness was found. Sleep- and wake-centered mechanisms for this relationship are discussed. Overall, this study suggests a central role of the thalamus in synaptic renormalization. ANN NEUROL 2021;90:821-833.
© 2021 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures

Similar articles
-
Thalamic Stroke and Sleep Study: Sleep-Wake, Autonomic Regulation, and Cognition.Stroke. 2025 Jun;56(6):1528-1541. doi: 10.1161/STROKEAHA.124.049156. Epub 2025 Mar 26. Stroke. 2025. PMID: 40135332
-
The estimation of excessive daytime sleepiness in post-stroke patients - a polysomnographic study.Respir Physiol Neurobiol. 2019 Sep;267:1-5. doi: 10.1016/j.resp.2019.05.013. Epub 2019 May 25. Respir Physiol Neurobiol. 2019. PMID: 31136826
-
Wake-up stroke: Clinical characteristics, sedentary lifestyle, and daytime sleepiness.Rev Assoc Med Bras (1992). 2016 Oct;62(7):628-634. doi: 10.1590/1806-9282.62.07.628. Rev Assoc Med Bras (1992). 2016. PMID: 27925041
-
Nonpharmacologic Management of Excessive Daytime Sleepiness.Sleep Med Clin. 2020 Jun;15(2):195-203. doi: 10.1016/j.jsmc.2020.02.018. Sleep Med Clin. 2020. PMID: 32386694 Review.
-
Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents.Pediatr Clin North Am. 2011 Jun;58(3):649-65. doi: 10.1016/j.pcl.2011.03.002. Epub 2011 Apr 1. Pediatr Clin North Am. 2011. PMID: 21600347 Free PMC article. Review.
Cited by
-
Sleep and Stroke: Opening Our Eyes to Current Knowledge of a Key Relationship.Curr Neurol Neurosci Rep. 2022 Nov;22(11):767-779. doi: 10.1007/s11910-022-01234-2. Epub 2022 Oct 3. Curr Neurol Neurosci Rep. 2022. PMID: 36190654 Free PMC article. Review.
-
Validation Study of the Richards-Campbell Sleep Questionnaire in Patients with Acute Stroke.J Pers Med. 2022 Sep 8;12(9):1473. doi: 10.3390/jpm12091473. J Pers Med. 2022. PMID: 36143258 Free PMC article.
References
-
- Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur J Neurol 2020;27:1117–1136. - PubMed
-
- Falck RS, Best JR, Davis JC, et al. Sleep and cognitive function in chronic stroke: a comparative cross‐sectional study. Sleep 2019;42::zsz040. - PubMed
Publication types
MeSH terms
Grants and funding
- 320030_153387/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- CRSII3_160803/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- CRPP Sleep and Health of Universität Zürich
- Funding Statement: Open access funding provided by Universitat Zurich.
- WOA Institution: Universitat Zurich

