Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis
- PMID: 34516020
- PMCID: PMC9292750
- DOI: 10.1111/aor.14066
Clinical assessment of liver metabolism during hypothermic oxygenated machine perfusion using microdialysis
Abstract
Background: While growing evidence supports the use of hypothermic oxygenated machine perfusion (HOPE) in liver transplantation, its effects on liver metabolism are still incompletely understood.
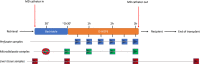
Methods: To assess liver metabolism during HOPE using microdialysis (MD), we conducted an open-label, observational pilot study on 10 consecutive grafts treated with dual-HOPE (D-HOPE). Microdialysate and perfusate levels of glucose, lactate, pyruvate, glutamate, and flavin mononucleotide (FMN) were measured during back table preparation and D-HOPE and correlated to graft function and patient outcome.
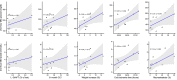
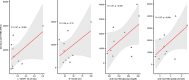
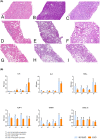
Results: Median (IQR) MD and D-HOPE time was 228 (210, 245) and 116 (103, 143) min. Three grafts developed early allograft dysfunction (EAD), with one requiring retransplantation. During D-HOPE, MD glucose and lactate levels increased (ANOVA = 9.88 [p = 0.01] and 3.71 [p = 0.08]). Their 2nd-hour levels were higher in EAD group and positively correlated with L-GrAFT score. 2nd-hour MD glucose and lactate were also positively correlated with cold ischemia time, macrovesicular steatosis, weight gain during D-HOPE, and perfusate FMN. These correlations were not apparent when perfusate levels were considered. In contrast, MD FMN levels invariably dropped steeply after D-HOPE start, whereas perfusate FMN was higher in dysfunctioning grafts.
Conclusion: MD glucose and lactate during D-HOPE are markers of hepatocellular injury and could represent additional elements of the viability assessment.
Keywords: extracellular fluid; flavin mononucleotide; liver metabolism; liver viability assessment; machine perfusion; microdialysis.
© 2021 The Authors. Artificial Organs published by International Center for Artificial Organ and Transplantation (ICAOT) and Wiley Periodicals LLC.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose.
Figures



References
-
- Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera MTPR, Isaac JR, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70:50–7. - PubMed
-
- van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. 2021;384:1391–401. - PubMed

