Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens
- PMID: 34516771
- PMCID: PMC8442908
- DOI: 10.1126/sciadv.abj2101
Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens
Abstract
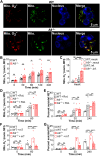
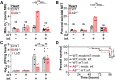
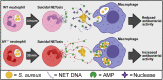
Neutrophils and macrophages are critical to the innate immune response, but cooperative mechanisms used by these cells to combat extracellular pathogens are not well understood. This study reveals that S100A9-deficient neutrophils produce higher levels of mitochondrial superoxide in response to Staphylococcus aureus and, as a result, form neutrophil extracellular traps (suicidal NETosis). Increased suicidal NETosis does not improve neutrophil killing of S. aureus in isolation but augments macrophage killing. NET formation enhances antibacterial activity by increasing phagocytosis by macrophages and by transferring neutrophil-specific antimicrobial peptides to them. Similar results were observed in response to other phylogenetically distinct bacterial pathogens including Streptococcus pneumoniae and Pseudomonas aeruginosa, implicating this as an immune defense mechanism that broadly enhances antibacterial activity. These results demonstrate that achieving maximal bactericidal activity through NET formation requires macrophages and that accelerated and more robust suicidal NETosis makes neutrophils adept at increasing antibacterial activity, especially when A9 deficient.
Figures
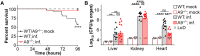






References
-
- Klein M., Wang A., Infective endocarditis. J. Intensive Care Med. 31, 151–163 (2016). - PubMed
-
- Collins L. V., Kristian S. A., Weidenmaier C., Faigle M., Van Kessel K. P., Van Strijp J. A., Gotz F., Neumeister B., Peschel A., Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186, 214–219 (2002). - PubMed
-
- Green J. N., Kettle A. J., Winterbourn C. C., Protein chlorination in neutrophil phagosomes and correlation with bacterial killing. Free Radic. Biol. Med. 77, 49–56 (2014). - PubMed
-
- Hampton M. B., Winterbourn C. C., Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radic. Biol. Med. 18, 633–639 (1995). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

