Chromosomal instability sensitizes patient breast tumors to multipolar divisions induced by paclitaxel
- PMID: 34516829
- PMCID: PMC8612166
- DOI: 10.1126/scitranslmed.abd4811
Chromosomal instability sensitizes patient breast tumors to multipolar divisions induced by paclitaxel
Abstract
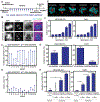
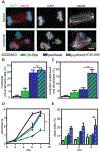
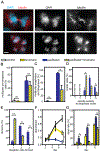
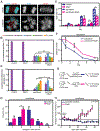
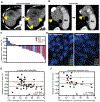
Paclitaxel (Taxol) is a cornerstone of cancer treatment. However, its mechanism of cytotoxicity is incompletely understood and not all patients benefit from treatment. We show that patients with breast cancer did not accumulate sufficient intratumoral paclitaxel to induce mitotic arrest in tumor cells. Instead, clinically relevant concentrations induced multipolar mitotic spindle formation. However, the extent of early multipolarity did not predict patient response. Whereas multipolar divisions frequently led to cell death, multipolar spindles focused into bipolar spindles before division at variable frequency, and maintaining multipolarity throughout mitosis was critical to induce the high rates of chromosomal instability necessary for paclitaxel to elicit cell death. Increasing multipolar divisions in paclitaxel resulted in improved cytotoxicity. Conversely, decreasing paclitaxel-induced multipolar divisions reduced paclitaxel efficacy. Moreover, we found that preexisting chromosomal instability sensitized breast cancer cells to paclitaxel. Both genetic and pharmacological methods of inducing chromosomal instability were sufficient to increase paclitaxel efficacy. In patients, the amount of pretreatment chromosomal instability directly correlated with taxane response in metastatic breast cancer such that patients with a higher rate of preexisting chromosomal instability showed improved response to taxanes. Together, these results support the use of baseline rates of chromosomal instability as a predictive biomarker for paclitaxel response. Furthermore, they suggest that agents that increase chromosomal instability or maintain multipolar spindles throughout mitosis will improve the clinical utility of paclitaxel.
Figures


References
-
- Huang TC, Campbell TC, Comparison of weekly versus every 3 weeks paclitaxel in the treatment of advanced solid tumors: a meta-analysis. Cancer Treat Rev 38, 613–617 (2012). - PubMed
-
- Crown J, O'Leary M, Ooi WS, Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 9 Suppl 2, 24–32 (2004). - PubMed
-
- Budd GT, Barlow WE, Moore HC, Hobday TJ, Stewart JA, Isaacs C, Salim M, Cho JK, Rinn KJ, Albain KS, Chew HK, Burton GV, Moore TD, Srkalovic G, McGregor BA, Flaherty LE, Livingston RB, Lew DL, Gralow JR, Hortobagyi GN, SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. J Clin Oncol 33, 58–64 (2015). - PMC - PubMed
-
- Fountzilas G, Dafni U, Dimopoulos MA, Koutras A, Skarlos D, Papakostas P, Gogas H, Bafaloukos D, Kalogera-Fountzila A, Samantas E, Briasoulis E, Pectasides D, Maniadakis N, Matsiakou F, Aravantinos G, Papadimitriou C, Karina M, Christodoulou C, Kosmidis P, Kalofonos HP, A randomized phase III study comparing three anthracycline-free taxane-based regimens, as first line chemotherapy, in metastatic breast cancer: a Hellenic Cooperative Oncology Group study. Breast Cancer Res Treat 115, 87–99 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

