The Clinical Application of Urine Soluble CD163 in ANCA-Associated Vasculitis
- PMID: 34518279
- PMCID: PMC8806104
- DOI: 10.1681/ASN.2021030382
The Clinical Application of Urine Soluble CD163 in ANCA-Associated Vasculitis
Abstract
Background: Up to 70% of patients with ANCA-associated vasculitis (AAV) develop GN, with 26% progressing to ESKD. Diagnostic-grade and noninvasive tools to detect active renal inflammation are needed. Urinary soluble CD163 (usCD163) is a promising biomarker of active renal vasculitis, but a diagnostic-grade assay, assessment of its utility in prospective diagnosis of renal vasculitis flares, and evaluation of its utility in proteinuric states are needed.
Methods: We assessed a diagnostic-grade usCD163 assay in (1) a real-world cohort of 405 patients with AAV and 121 healthy and 488 non-AAV disease controls; (2) a prospective multicenter study of 84 patients with potential renal vasculitis flare; (3) a longitudinal multicenter cohort of 65 patients with podocytopathy; and (4) a cohort of 29 patients with AAV (with or without proteinuria) and ten controls.
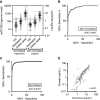
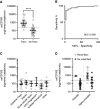
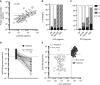
Results: We established a diagnostic reference range, with a cutoff of 250 ng/mmol for active renal vasculitis (area under the curve [AUC], 0.978). Using this cutoff, usCD163 was elevated in renal vasculitis flare (AUC, 0.95) but remained low in flare mimics, such as nonvasculitic AKI. usCD163's specificity declined in patients with AAV who had nephrotic-range proteinuria and in those with primary podocytopathy, with 62% of patients with nephrotic syndrome displaying a "positive" usCD163. In patients with AAV and significant proteinuria, usCD163 normalization to total urine protein rather than creatinine provided the greatest clinical utility for diagnosing active renal vasculitis.
Conclusions: usCD163 is elevated in renal vasculitis flare and remains low in flare mimics. Nonspecific protein leakage in nephrotic syndrome elevates usCD163 in the absence of glomerular macrophage infiltration, resulting in false-positive results; this can be corrected with urine protein normalization.
Keywords: ANCA; CD163; biomarker; crescentic glomerulonephritis; macrophage; urine.
Copyright © 2021 by the American Society of Nephrology.
Figures


References
-
- Jennette JC, Olson JL, Schwartz MM, Silva FG, Thomas DB: Pauci-immune and antineutrophil cytoplasmic autoantibody–mediated crescentic glomerulonephritis and vasculitis. In: Heptinstall’s Pathology of the Kidney, edited by Olson JL, Jennette JC, Schwartz MM, Silva FG, Philadelphia, Lippincott Williams and Wilkins, 2007, pp 643–674
-
- Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. ; Pan-Thames Renal Research Group : Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 41: 776–784, 2003 - PubMed
-
- Mohammad AJ, Segelmark M: A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 41: 1366–1373, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

