RORγt Promotes Foxp3 Expression by Antagonizing the Effector Program in Colonic Regulatory T Cells
- PMID: 34518282
- PMCID: PMC8490938
- DOI: 10.4049/jimmunol.2100175
RORγt Promotes Foxp3 Expression by Antagonizing the Effector Program in Colonic Regulatory T Cells
Abstract
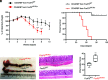
RORγt is the master transcription factor for the Th17 cells. Paradoxically, in the intestine, RORγt is coexpressed in peripherally induced regulatory T cells (pTregs) together with Foxp3, the master transcription factor for Tregs. Unexpectedly, by an unknown mechanism, colonic RORγt+ Tregs show an enhanced suppressor function and prevent intestinal inflammation more efficiently than RORγt-nonexpressing pTregs. Although studies have elucidated the function of RORγt in Th17 cells, how RORγt regulates pTreg function is not understood. In our attempt to understand the role of RORγt in controlling Treg function, we discovered a RORγt-driven pathway that modulates the regulatory (suppressor) function of colonic Tregs. We found that RORγt plays an essential role in maintaining Foxp3 expression. RORγt-deficient Tregs failed to sustain Foxp3 expression with concomitant upregulation of T-bet and IFN-γ expressions. During colitis induced by adoptive transfer of CD45RBhi cells in Rag1 -/- mice, RORγt-deficient colonic Tregs transitioned to a Th1-like effector phenotype and lost their suppressor function, leading to severe colitis with significant mortality. Accordingly, Foxp3-expressing, RORγt-deficient Tregs showed impaired therapeutic efficacy in ameliorating colitis that is not due to their reduced survival. Moreover, using the Treg-specific RORγt and T-bet double-deficient gene knockout mouse, we demonstrate that deletion of T-bet from RORγt-deficient Tregs restored Foxp3 expression and suppression function as well as prevented onset of severe colitis. Mechanistically, our study suggests that RORγt-mediated repression of T-bet is critical to regulating the immunosuppressive function of colonic Tregs during the inflammatory condition.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures







References
-
- Sakaguchi S., Vignali D. A., Rudensky A. Y., Niec R. E., Waldmann H.. 2013. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13: 461–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

