Axon Regeneration: A Subcellular Extension in Multiple Dimensions
- PMID: 34518340
- PMCID: PMC8886981
- DOI: 10.1101/cshperspect.a040923
Axon Regeneration: A Subcellular Extension in Multiple Dimensions
Abstract
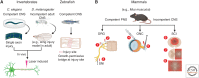
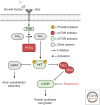
Axons are a unique cellular structure that allows for the communication between neurons. Axon damage compromises neuronal communications and often leads to functional deficits. Thus, developing strategies that promote effective axon regeneration for functional restoration is highly desirable. One fruitful approach is to dissect the regenerative mechanisms used by some types of neurons in both mammalian and nonmammalian systems that exhibit spontaneous regenerative capacity. Additionally, numerous efforts have been devoted to deciphering the barriers that prevent successful axon regeneration in the most regeneration-refractory system-the adult mammalian central nervous system. As a result, several regeneration-promoting strategies have been developed, but significant limitations remain. This review is aimed to summarize historic progression and current understanding of this exciting yet incomplete endeavor.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
