Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy
- PMID: 34518515
- PMCID: PMC8437938
- DOI: 10.1038/s41421-021-00299-6
Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy
Abstract
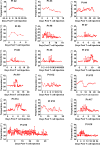
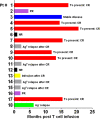
IL6 is one of the most elevated cytokines during chimeric antigen receptor (CAR) T cell cytokine release syndrome (CRS), and IL6R blockade by Tocilizumab has successfully relieved the most life-threatening aspects of CRS in patients. In addition, latest studies demonstrated the essential role of IL1 in driving CART induced neurotoxicity in mouse models. Here we present a clinical investigation (ChiCTR2000032124; ChiCTR2000031868) of anti-CD19 and anti-BCMA CART (41BBζ) secreting an anti-IL6 scFv and IL1 receptor antagonist (IL1RA) in treating patients with hematologic malignancy. Our results revealed that IL6 and IL1B were maintained at low levels without significant elevation during CRS, rendering Tocilizumab dispensable. Moreover, treated patients did not show neurotoxicity during CRS and exhibited mild to moderate CRS. Notably, we observed high rate of complete response (CR) and significant CART expansion during treatment. In sum, we conclude that CART-secreting anti-IL6 scFv and IL1RA could self-neutralize IL6 storm and maintain low levels of IL1B during CART therapy to minimize IL6- and IL1-associated cytokine toxicity and neurotoxicity without impairing therapeutic efficacy.
© 2021. The Author(s).
Conflict of interest statement
All IP rights surrounding the technology in designing and engineering the CART cells used in this study belong to Celledit and Siweikang Therapeutics. B.H. is the founder of Celledit and Siweikang Therapeutics. The other authors do not have any financial interest to disclose.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

