Relief of excited-state antiaromaticity enables the smallest red emitter
- PMID: 34518551
- PMCID: PMC8438045
- DOI: 10.1038/s41467-021-25677-2
Relief of excited-state antiaromaticity enables the smallest red emitter
Abstract
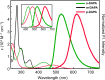
It is commonly accepted that a large π-conjugated system is necessary to realize low-energy electronic transitions. Contrary to this prevailing notion, we present a new class of light-emitters utilizing a simple benzene core. Among different isomeric forms of diacetylphenylenediamine (DAPA), o- and p-DAPA are fluorescent, whereas m-DAPA is not. Remarkably, p-DAPA is the lightest (FW = 192) molecule displaying red emission. A systematic modification of the DAPA system allows the construction of a library of emitters covering the entire visible color spectrum. Theoretical analysis shows that their large Stokes shifts originate from the relief of excited-state antiaromaticity, rather than the typically assumed intramolecular charge transfer or proton transfer. A delicate interplay of the excited-state antiaromaticity and hydrogen bonding defines the photophysics of this new class of single benzene fluorophores. The formulated molecular design rules suggest that an extended π-conjugation is no longer a prerequisite for a long-wavelength light emission.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Liu YC, Li CS, Ren ZJ, Yan SK, Bryce MR. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mat. 2018;3:18020. doi: 10.1038/natrevmats.2018.20. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

