This is a preprint.
Optimal strategies to screen health care workers for COVID-19 in the US: a cost-effectiveness analysis
- PMID: 34518835
- PMCID: PMC8437316
- DOI: 10.21203/rs.3.rs-887590/v1
Optimal strategies to screen health care workers for COVID-19 in the US: a cost-effectiveness analysis
Update in
-
Optimal strategies to screen health care workers for COVID-19 in the US: a cost-effectiveness analysis.Cost Eff Resour Alloc. 2022 Jan 15;20(1):2. doi: 10.1186/s12962-021-00336-x. Cost Eff Resour Alloc. 2022. PMID: 35033100 Free PMC article.
Abstract
Background: Transmission of SARS-CoV-2 in health care facilities poses a challenge against pandemic control. Health care workers (HCWs) have frequent and high-risk interactions with COVID-19 patients. We undertook a cost-effectiveness analysis to determine optimal testing strategies for screening HCWs to inform strategic decision-making in health care settings.
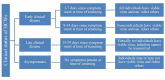
Methods: We modeled the number of new infections, quality-adjusted life years lost, and net costs related to six testing strategies including no tests. We applied our model to four strata of HCWs, defined by the presence and timing of symptoms. We conducted sensitivity analyses to account for uncertainty in inputs.
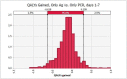
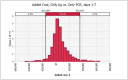
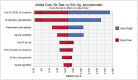
Results: When screening recently symptomatic HCWs, conducting only a PCR test is preferable; it saves costs and improves health outcomes in the first week post-symptom onset, and costs $83,000 per quality-adjusted life year gained in the second week post-symptom onset. When screening HCWs in the late clinical disease stage, none of the testing approaches is cost-effective and thus no testing is preferable, yielding $11 and 0.003 new infections per 10 HCWs. For screening asymptomatic HCWs, antigen testing is preferable to PCR testing due to its lower cost.
Conclusions: Both PCR and antigen testing are beneficial strategies to identify infected HCWs and reduce transmission of SARS-CoV-2 in health care settings. IgG testing clinical value depends on test timing and immunity characteristics, however is not cost-effective in a low prevalence setting. As the context of the pandemic evolves, our study provides insight to health-care decision makers to keep the health care workforce safe and transmissions low.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures
References
-
- Pneumonia of unknown cause - China [Internet], WHO. World Health Organization; 2020. [cited 2020 May 20]. Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch...
-
- Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020Feb25;323(8):709–10. - PubMed
-
- COVID-19 Dashboard by CSSE at Johns Hopkins University [Internet]. 2020. [cited 2020 Jun 12]. Available from: https://coronavirus.jhu.edu/map.html
-
- NHS. SARS (severe acute respiratory syndrome) [Internet]. 2019. [cited 2020 Jun 12]. Available from: https://www.nhs.uk/conditions/sars/
-
- Taylor DB. How the Coronavirus Pandemic Unfolded: a Timeline. The New York Times; [Internet]. 2020May12 [cited 2020 May 21]; Available from: https://www.nytimes.com/article/coronavirus-timeline.html
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

