Evidence for tetrodotoxin-resistant spontaneous myogenic contractions of mouse isolated stomach that are dependent on acetylcholine
- PMID: 34519057
- PMCID: PMC9297954
- DOI: 10.1111/bph.15685
Evidence for tetrodotoxin-resistant spontaneous myogenic contractions of mouse isolated stomach that are dependent on acetylcholine
Abstract
Background and purpose: Gastric pacemaker cells, interstitial cells of Cajal (ICC), are believed to initiate myogenic (non-neuronal) contractions. These become damaged in gastroparesis, associated with dysrhythmic electrical activity and nausea. We utilised mouse isolated stomach to model myogenic contractions and investigate their origin and actions of interstitial cells of Cajal modulators.
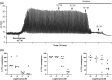
Experimental approach: Intraluminal pressure was recorded following distension with a physiological volume; tone, contraction amplitude and frequency were quantified. Compounds were bath applied.
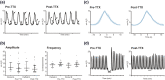
Key results: The stomach exhibited regular large amplitude contractions (median amplitude 9.0 [4.7-14.8] cmH2 O, frequency 2.9 [2.5-3.4] c.p.m; n = 20), appearing to progress aborally. Tetrodotoxin (TTX, 10-6 M) had no effect on tone, frequency or amplitude but blocked responses to nerve stimulation. ω-conotoxin GVIA (10-7 M) ± TTX was without effect on baseline motility. In the presence of TTX, (1) atropine (10-10 -10-6 M) reduced contraction amplitude and frequency in a concentration-related manner (pIC50 7.5 ± 0.3 M for amplitude), (2) CaCC channel (previously ANO1) inhibitors MONNA and CaCCinh-A01 reduced contraction amplitude (significant at 10-5 , 10-4 M respectively) and frequency (significant at 10-5 M), and (3), neostigmine (10-5 M) evoked a large, variable, increase in contraction amplitude, reduced by atropine (10-8 -10-6 M) but unaffected (exploratory study) by the H1 receptor antagonist mepyramine (10-6 M).
Conclusions and implications: The distended mouse stomach exhibited myogenic contractions, resistant to blockade of neural activity by TTX. In the presence of TTX, these contractions were prevented or reduced by compounds blocking interstitial cells of Cajal activity or by atropine and enhanced by neostigmine (antagonised by atropine), suggesting involvement of non-neuronal ACh in their regulation.
Keywords: ACh; ANO1; CaCC; interstitial cells of Cajal; myogenic contractions; nausea; stomach.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
WC, MS, RM, AG and PLRA declare no potential conflict of interest. GJS receives a research grant from Takeda Pharmaceuticals.
Figures




References
-
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. M. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , Catterall, W. A. , … CGTP collaborators . (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Ion channels. British Journal of Pharmacology, 178, S157–S245. 10.1111/bph.15539 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

