Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial
- PMID: 34519801
- PMCID: PMC8441593
- DOI: 10.1001/jama.2021.12836
Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial
Abstract
Importance: Standard first-line therapy for advanced or metastatic esophageal carcinoma is chemotherapy, but the prognosis remains poor. Camrelizumab (an anti-programmed death receptor 1 [PD-1] antibody) showed antitumor activity in previously treated advanced or metastatic esophageal squamous cell carcinoma.
Objective: To evaluate the efficacy and adverse events of camrelizumab plus chemotherapy vs placebo plus chemotherapy as a first-line treatment in advanced or metastatic esophageal squamous cell carcinoma.
Design, setting, and participants: This randomized, double-blind, placebo-controlled, multicenter, phase 3 trial (ESCORT-1st study) enrolled patients from 60 hospitals in China between December 3, 2018, and May 12, 2020 (final follow-up, October 30, 2020). A total of 751 patients were screened and 596 eligible patients with untreated advanced or metastatic esophageal squamous cell carcinoma were randomized.
Interventions: Patients were randomized 1:1 to receive either camrelizumab 200 mg (n = 298) or placebo (n = 298), combined with up to 6 cycles of paclitaxel (175 mg/m2) and cisplatin (75 mg/m2). All treatments were given intravenously every 3 weeks.
Main outcomes and measures: Coprimary end points were overall survival (significance threshold, 1-sided P < .02) and progression-free survival (significance threshold, 1-sided P < .005).
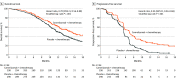
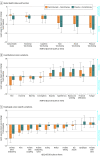
Results: Of the 596 patients randomized (median age, 62 years [interquartile range, 56-67 years]; 523 men [87.8%]), 1 patient in the placebo-chemotherapy group did not receive planned treatment. A total of 490 patients (82.2%) had discontinued the study treatment. The median follow-up was 10.8 months. The overall survival for the camrelizumab-chemotherapy group was a median of 15.3 months (95% CI, 12.8-17.3; 135 deaths) vs a median of 12.0 months (95% CI, 11.0-13.3; 174 deaths) for the placebo-chemotherapy group (hazard ratio [HR] for death, 0.70 [95% CI, 0.56-0.88]; 1-sided P = .001). Progression-free survival for camrelizumab plus chemotherapy was a median of 6.9 months (95% CI, 5.8-7.4; 199 progression or deaths) vs 5.6 months (95% CI, 5.5-5.7; 229 progression or deaths) for the placebo-chemotherapy group (HR for progression or death, 0.56 [95% CI, 0.46-0.68]; 1-sided P < .001). Treatment-related adverse events of grade 3 or higher occurred in 189 patients (63.4%) in the camrelizumab-chemotherapy group and 201 (67.7%) in the placebo-chemotherapy group, including treatment-related deaths among 9 patients (3.0%) and 11 patients (3.7%), respectively.
Conclusions and relevance: Among patients with advanced or metastatic esophageal squamous cell carcinoma, the addition of camrelizumab to chemotherapy, compared with placebo and chemotherapy, significantly improved overall survival and progression-free survival.
Trial registration: ClinicalTrials.gov Identifier: NCT03691090.
Conflict of interest statement
Figures
Comment in
-
Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma-Renewed Enthusiasm and a Lingering Challenge.JAMA Oncol. 2021 Nov 1;7(11):1613-1614. doi: 10.1001/jamaoncol.2021.4410. JAMA Oncol. 2021. PMID: 34519775 No abstract available.
References
-
- National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers. NCCN Clinical Practice Guidelines in Oncology. Version 2. Published March 9, 2021. Accessed May 5, 2021. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf

