Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication
- PMID: 34519916
- PMCID: PMC8692537
- DOI: 10.1007/s12250-021-00433-4
Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication
Abstract
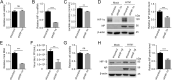
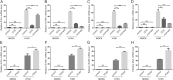
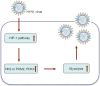
Viruses depend on host cellular metabolism to provide the energy and biosynthetic building blocks required for their replication. In this study, we observed that influenza A virus (H1N1), a single-stranded, negative-sense RNA virus with an eight-segmented genome, enhanced glycolysis both in mouse lung tissues and in human lung epithelial (A549) cells. In detail, the expression of hexokinase 2 (HK2), the first enzyme in glycolysis, was upregulated in H1N1-infected A549 cells, and the expression of pyruvate kinase M2 (PKM2) and pyruvate dehydrogenase kinase 3 (PDK3) was upregulated in H1N1-infected mouse lung tissues. Pharmacologically inhibiting the glycolytic pathway or targeting hypoxia-inducible factor 1 (HIF-1), the central transcriptional factor critical for glycolysis, significantly reduced H1N1 replication, revealing a requirement for glycolysis during H1N1 infection. In addition, pharmacologically enhancing the glycolytic pathway further promoted H1N1 replication. Furthermore, the change of H1N1 replication upon glycolysis inhibition or enhancement was independent of interferon signaling. Taken together, these findings suggest that influenza A virus induces the glycolytic pathway and thus facilitates efficient viral replication. This study raises the possibility that metabolic inhibitors, such as those that target glycolysis, could be used to treat influenza A virus infection in the future.
Keywords: Glycolysis; H1N1; Hypoxia-inducible factor 1 (HIF-1); Interferon; Replication.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Asim M, Jiang S, Yi L, Chen W, Sun L, Zhao L, Khan Khattak MN, Tu J, Lin L. Glutamine is required for red-spotted grouper nervous necrosis virus replication via replenishing the tricarboxylic acid cycle. Virus Res. 2017;227:245–248. - PubMed
-
- Baker JC, Yan X, Peng T, Kasten S, Roche TE. Marked differences between two isoforms of human pyruvate dehydrogenase kinase. J Biol Chem. 2000;275:15773–15781. - PubMed
-
- Barban S, Schulze HO. The effects of 2-deoxyglucose on the growth and metabolism of cultured human cells. J Biol Chem. 1961;236:1887–1890. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

