The therapeutic potential of adipose tissue-derived mesenchymal stromal cells in the treatment of busulfan-induced azoospermic mice
- PMID: 34519944
- PMCID: PMC8866597
- DOI: 10.1007/s10815-021-02309-8
The therapeutic potential of adipose tissue-derived mesenchymal stromal cells in the treatment of busulfan-induced azoospermic mice
Abstract
Purpose: The generation of germ cells from mesenchymal stromal cells (MSCs) provides a valuable in vitro platform for infertility modeling. The establishment of these cells is a new approach for assisted reproductive technology (ART) to help infertile patients who lack functional gametes.
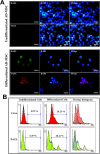
Methods: Human adipose-derived MSCs were isolated and then characterized for multipotency by flow cytometry, differentiation capacity, and cytogenetic assays. These cells were used in a male germ cell differentiation study. The expression of male germ cell markers was evaluated at day 21 of differentiation using an immunofluorescence assay, flow cytometry, and RT-qPCR. Undifferentiated MSCs were used for transplantation in busulfan-induced azoospermic mice.
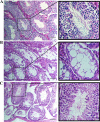
Results: In this study, MSCs were successfully isolated from human adipose tissues which were positive for cell markers such as CD90, CD105, CD73, and CD29 but negative for CD34 and CD45. The results of flow cytometry, immunocytochemistry, and RT-qPCR analysis at day 21 of differentiation showed that the undifferentiated adipose-derived MSCs are able to differentiate into male germ cells. Additionally, transplantation of undifferentiated MSCs in busulfan-induced azoospermic mice caused spermatogenesis recovery in the majority of seminiferous tubules.
Conclusion: In this study, we showed that differentiation of human adipose-derived MSCs into male germ cells is a useful tool for in vitro study of human germ cell development. Our results demonstrated that cell therapy with adipose-derived MSCs could help the repair of pathological changes in testicular seminiferous tubules. Therefore, it may have a clinical application for the treatment of azoospermia in infertile patients.
Keywords: Adipose tissue; Differentiation; Germ cell; Mesenchymal cells.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Enhancing Spermatogenesis in Non-obstructive Azoospermia Through Mesenchymal Stem Cell Therapy22.Curr Stem Cell Res Ther. 2024;19(11):1429-1441. doi: 10.2174/011574888X283311231226081845. Curr Stem Cell Res Ther. 2024. PMID: 38243988 Review.
-
Successful transplantation of spermatogonial stem cells into the seminiferous tubules of busulfan-treated mice.Reprod Health. 2021 Sep 23;18(1):189. doi: 10.1186/s12978-021-01242-4. Reprod Health. 2021. PMID: 34556135 Free PMC article.
-
Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats.J Hum Reprod Sci. 2015 Apr-Jun;8(2):103-10. doi: 10.4103/0974-1208.158618. J Hum Reprod Sci. 2015. PMID: 26157302 Free PMC article.
-
Altered expression of some miRNAs and their target genes following mesenchymal stem cell treatment in busulfan-induced azoospermic rats.Gene. 2020 May 5;737:144481. doi: 10.1016/j.gene.2020.144481. Epub 2020 Feb 15. Gene. 2020. PMID: 32070749
-
The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation.Stem Cell Rev Rep. 2014 Feb;10(1):79-85. doi: 10.1007/s12015-013-9478-8. Stem Cell Rev Rep. 2014. PMID: 24142483 Free PMC article. Review.
Cited by
-
Enhancing Spermatogenesis in Non-obstructive Azoospermia Through Mesenchymal Stem Cell Therapy22.Curr Stem Cell Res Ther. 2024;19(11):1429-1441. doi: 10.2174/011574888X283311231226081845. Curr Stem Cell Res Ther. 2024. PMID: 38243988 Review.
-
Preventing/Reversing Adverse Effects of Endocrine Disruption on Mouse Testes by Normalizing Tissue Resident VSELs.Stem Cell Rev Rep. 2023 Oct;19(7):2525-2540. doi: 10.1007/s12015-023-10601-6. Epub 2023 Aug 10. Stem Cell Rev Rep. 2023. PMID: 37561284
-
Effects of clinical medications on male fertility and prospects for stem cell therapy.Front Cell Dev Biol. 2023 Sep 18;11:1258574. doi: 10.3389/fcell.2023.1258574. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37791073 Free PMC article. Review.
-
Current Progress in Stem Cell Therapy for Male Infertility.Stem Cell Rev Rep. 2023 Oct;19(7):2073-2093. doi: 10.1007/s12015-023-10577-3. Epub 2023 Jul 13. Stem Cell Rev Rep. 2023. PMID: 37440145 Review.
References
-
- Zhang H, et al. Translocation breakpoints of chromosome 3 in male carriers: a report of twelve cases and a review of the literature. Turk J Med Sci. 2018;48(1):150–156. - PubMed
-
- Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod Biomed Online. 2014;29(2):150–155. - PubMed
-
- Hayashi K et al. Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Science. 2012;1222482. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

