UPF1 impacts on mTOR signaling pathway and autophagy in endometrioid endometrial carcinoma
- PMID: 34520393
- PMCID: PMC8457587
- DOI: 10.18632/aging.203421
UPF1 impacts on mTOR signaling pathway and autophagy in endometrioid endometrial carcinoma
Abstract
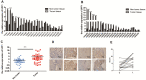
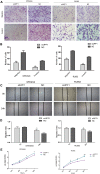
Most EEC cases are associated with activities of the mTOR pathway, which regulates protein synthesis, cell growth and autophagy. While Up-Frameshift 1(UPF1) is a key protein factor in the nonsense-mediated mRNA degradation pathway (NMD), its role in carcinogenesis of EEC remains unclear. In this study, we first evaluated the expression level of UPF1 in EEC tissues and cell lines. Then, we investigated the effect of UPF1 on cellular function and mTOR signaling pathway; these effects were further validated in vivo. Finally, its effect on autophagy was evaluated by western blot and GFP-mRFP-LC3 staining. UPF1 expression in the EEC tissue samples was significantly higher than that of matched normal tissue samples. Overexpression of UPF1 promoted migration and invasion of EEC cells. Conversely, depletion of UPF1 suppressed migration and invasion of EEC cells. In addition, overexpression of UPF1 increased the in vivo growth of our EEC xenograft tumors. Finally, UPF1 increased the activity of the mTOR/P70S6K/4EBP1 signaling pathway and inhibited autophagy in EEC cells. These findings suggest that UPF1 functions as an oncogene to promote EEC carcinogenesis. Our findings propose UPF1 as a new potential therapeutic target for EEC.
Keywords: UPF1; autophagy; endometrioid endometrial carcinoma; mTOR.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

