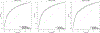Machine Learning Algorithms Using Routinely Collected Data Do Not Adequately Predict Viremia to Inform Targeted Services in Postpartum Women Living With HIV
- PMID: 34520443
- PMCID: PMC8585692
- DOI: 10.1097/QAI.0000000000002800
Machine Learning Algorithms Using Routinely Collected Data Do Not Adequately Predict Viremia to Inform Targeted Services in Postpartum Women Living With HIV
Abstract
Background: Adherence to antiretroviral treatment (ART) among postpartum women with HIV is essential for optimal health and prevention of perinatal transmission. However, suboptimal adherence with subsequent viremia is common, and adherence challenges are often underreported. We aimed to predict viremia to facilitate targeted adherence support in sub-Saharan Africa during this critical period.
Methods: Data are from PROMISE 1077BF/FF, which enrolled perinatal women between 2011 and 2014. This analysis includes postpartum women receiving ART per study randomization or country-specific criteria to continue from pregnancy. We aimed to predict viremia (single and confirmed events) after 3 months on ART at >50, >400, and >1000 copies/mL within 6-month intervals through 24 months. We built models with routine clinical and demographic data using the least absolute shrinkage and selection operator and SuperLearner (which incorporates multiple algorithms).
Results: Among 1321 women included, the median age was 26 years and 96% were in WHO stage 1. Between 0 and 24 months postpartum, 42%, 31%, and 28% of women experienced viremia >50, >400, and >1000 copies/mL, respectively, at least once. Across models, the cross-validated area under the receiver operating curve ranged from 0.74 [95% confidence interval (CI): 0.72 to 0.76] to 0.78 (95% CI: 0.76 to 0.80). To achieve 90% sensitivity predicting confirmed viremia >50 copies/mL, 64% of women would be classified as high risk.
Conclusions: Using routinely collected data to predict viremia in >1300 postpartum women with HIV, we achieved moderate model discrimination, but insufficient to inform targeted adherence support. Psychosocial characteristics or objective adherence metrics may be required for improved prediction of viremia in this population.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- UNAIDS. Start Free, Stay Free, AIDS Free: Final report on 2020 targets. Geneva, Switzerland, 2021.
-
- Flynn PM, Taha TE, Cababasay M, et al. Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): A randomized, open-label, clinical trial. J Acquir Immune Defic Syndr. 2018;77(4):383–392. - PMC - PubMed