Tuberculosis preventive therapy for people living with HIV: A systematic review and network meta-analysis
- PMID: 34520459
- PMCID: PMC8439495
- DOI: 10.1371/journal.pmed.1003738
Tuberculosis preventive therapy for people living with HIV: A systematic review and network meta-analysis
Abstract
Background: Tuberculosis (TB) preventive therapy (TPT) is an essential component of care for people living with HIV (PLHIV). We compared efficacy, safety, completion, and drug-resistant TB risk for currently recommended TPT regimens through a systematic review and network meta-analysis (NMA) of randomized trials.
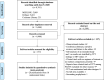
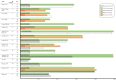
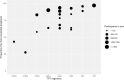
Methods and findings: We searched MEDLINE, Embase, and the Cochrane Library from inception through June 9, 2020 for randomized controlled trials (RCTs) comparing 2 or more TPT regimens (or placebo/no treatment) in PLHIV. Two independent reviewers evaluated eligibility, extracted data, and assessed the risk of bias. We grouped TPT strategies as follows: placebo/no treatment, 6 to 12 months of isoniazid, 24 to 72 months of isoniazid, and rifamycin-containing regimens. A frequentist NMA (using graph theory) was carried out for the outcomes of development of TB disease, all-cause mortality, and grade 3 or worse hepatotoxicity. For other outcomes, graphical descriptions or traditional pairwise meta-analyses were carried out as appropriate. The potential role of confounding variables for TB disease and all-cause mortality was assessed through stratified analyses. A total of 6,466 unique studies were screened, and 157 full texts were assessed for eligibility. Of these, 20 studies (reporting 16 randomized trials) were included. The median sample size was 616 (interquartile range [IQR], 317 to 1,892). Eight were conducted in Africa, 3 in Europe, 3 in the Americas, and 2 included sites in multiple continents. According to the NMA, 6 to 12 months of isoniazid were no more efficacious in preventing microbiologically confirmed TB than rifamycin-containing regimens (incidence rate ratio [IRR] 1.0, 95% CI 0.8 to 1.4, p = 0.8); however, 6 to 12 months of isoniazid were associated with a higher incidence of all-cause mortality (IRR 1.6, 95% CI 1.2 to 2.0, p = 0.02) and a higher risk of grade 3 or higher hepatotoxicity (risk difference [RD] 8.9, 95% CI 2.8 to 14.9, p = 0.004). Finally, shorter regimens were associated with higher completion rates relative to longer regimens, and we did not find statistically significant differences in the risk of drug-resistant TB between regimens. Study limitations include potential confounding due to differences in posttreatment follow-up time and TB incidence in the study setting on the estimates of incidence of TB or all-cause mortality, as well as an underrepresentation of pregnant women and children.
Conclusions: Rifamycin-containing regimens appear safer and at least as effective as isoniazid regimens in preventing TB and death and should be considered part of routine care in PLHIV. Knowledge gaps remain as to which specific rifamycin-containing regimen provides the optimal balance of efficacy, completion, and safety.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. Epub 2015/08/13. doi: 10.1097/QAD.0000000000000802 ; PubMed Central PMCID: PMC4568896. - DOI - PMC - PubMed
-
- World Health Organization. Global tuberculosis report 2020. Geneva: 2020.
-
- World Health Organization. Latent TB Infection: Updated and consolidated guidelines for programmatic management. 2018. . - PubMed
-
- Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11. Epub 2020/02/14. doi: 10.15585/mmwr.rr6901a1 ; PubMed Central PMCID: PMC7041302 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

