Economic and modeling evidence for tuberculosis preventive therapy among people living with HIV: A systematic review and meta-analysis
- PMID: 34520463
- PMCID: PMC8439468
- DOI: 10.1371/journal.pmed.1003712
Economic and modeling evidence for tuberculosis preventive therapy among people living with HIV: A systematic review and meta-analysis
Abstract
Background: Human immunodeficiency virus (HIV) is the strongest known risk factor for tuberculosis (TB) through its impairment of T-cell immunity. Tuberculosis preventive treatment (TPT) is recommended for people living with HIV (PLHIV) by the World Health Organization, as it significantly reduces the risk of developing TB disease. We conducted a systematic review and meta-analysis of modeling studies to summarize projected costs, risks, benefits, and impacts of TPT use among PLHIV on TB-related outcomes.
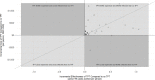
Methods and findings: We searched MEDLINE, Embase, and Web of Science from inception until December 31, 2020. Two reviewers independently screened titles, abstracts, and full texts; extracted data; and assessed quality. Extracted data were summarized using descriptive analysis. We performed quantile regression and random effects meta-analysis to describe trends in cost, effectiveness, and cost-effectiveness outcomes across studies and identified key determinants of these outcomes. Our search identified 6,615 titles; 61 full texts were included in the final review. Of the 61 included studies, 31 reported both cost and effectiveness outcomes. A total of 41 were set in low- and middle-income countries (LMICs), while 12 were set in high-income countries (HICs); 2 were set in both. Most studies considered isoniazid (INH)-based regimens 6 to 2 months long (n = 45), or longer than 12 months (n = 11). Model parameters and assumptions varied widely between studies. Despite this, all studies found that providing TPT to PLHIV was predicted to be effective at averting TB disease. No TPT regimen was substantially more effective at averting TB disease than any other. The cost of providing TPT and subsequent downstream costs (e.g. post-TPT health systems costs) were estimated to be less than $1,500 (2020 USD) per person in 85% of studies that reported cost outcomes (n = 36), regardless of study setting. All cost-effectiveness analyses concluded that providing TPT to PLHIV was potentially cost-effective compared to not providing TPT. In quantitative analyses, country income classification, consideration of antiretroviral therapy (ART) use, and TPT regimen use significantly impacted cost-effectiveness. Studies evaluating TPT in HICs suggested that TPT may be more effective at preventing TB disease than studies evaluating TPT in LMICs; pooled incremental net monetary benefit, given a willingness-to-pay threshold of country-level per capita gross domestic product (GDP), was $271 in LMICs (95% confidence interval [CI] -$81 to $622, p = 0.12) and was $2,568 in HICs (-$32,115 to $37,251, p = 0.52). Similarly, TPT appeared to be more effective at averting TB disease in HICs; pooled percent reduction in active TB incidence was 20% (13% to 27%, p < 0.001) in LMICs and 37% (-34% to 100%, p = 0.13) in HICs. Key limitations of this review included the heterogeneity of input parameters and assumptions from included studies, which limited pooling of effect estimates, inconsistent reporting of model parameters, which limited sample sizes of quantitative analyses, and database bias toward English publications.
Conclusions: The body of literature related to modeling TPT among PLHIV is large and heterogeneous, making comparisons across studies difficult. Despite this variability, all studies in all settings concluded that providing TPT to PLHIV is potentially effective and cost-effective for preventing TB disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO consolidated guidelines on tuberculosis. Geneva: World Health Organization; 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

