Reversible Dissociation of a Dialumene*
- PMID: 34520616
- PMCID: PMC8596890
- DOI: 10.1002/anie.202111385
Reversible Dissociation of a Dialumene*
Abstract
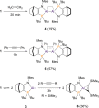
Dialumenes are neutral AlI compounds with Al=Al multiple bonds. We report the isolation of an amidophosphine-supported dialumene. Our X-ray crystallographic, spectroscopic, and computational DFT analyses reveal a long and extreme trans-bent Al=Al bond with a low dissociation energy and bond order. In solution, the dialumene can dissociate into monomeric AlI species. Reactivity studies reveal two modes of reaction: as dialumene or as aluminyl monomers.
Keywords: aluminium; aluminium(I) compounds; dialumene; low-valent atoms; multiple bonds.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

