PDE1 Inhibition Modulates Cav1.2 Channel to Stimulate Cardiomyocyte Contraction
- PMID: 34521216
- PMCID: PMC8553000
- DOI: 10.1161/CIRCRESAHA.121.319828
PDE1 Inhibition Modulates Cav1.2 Channel to Stimulate Cardiomyocyte Contraction
Abstract
[Figure: see text].
Keywords: excitation contraction coupling; myocardial contraction; nucleotide; pharmacology; phosphorylation.
Figures





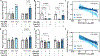
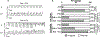

References
-
- Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo-Leiro MG, Dahlström U, Echeverria LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Li J, Lund M, Macdonald P, Mareev V, Momomura SI, O’Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Varin C, Honarpour N, Abbasi SA, Malik FI, Kurtz CE. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116 - PubMed
-
- Movsesian MA, Bristow MR. Alterations in camp-mediated signaling and their role in the pathophysiology of dilated cardiomyopathy. Curr.Top.Dev.Biol 2005;68:25–48 - PubMed
-
- Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM, Investigators O-C. Heart failure etiology and response to milrinone in decompensated heart failure: Results from the optime-chf study. J Am Coll Cardiol. 2003;41:997–1003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

