Safety and Efficacy of Natalizumab as Adjunctive Therapy for People With Drug-Resistant Epilepsy: A Phase 2 Study
- PMID: 34521687
- PMCID: PMC8610627
- DOI: 10.1212/WNL.0000000000012766
Safety and Efficacy of Natalizumab as Adjunctive Therapy for People With Drug-Resistant Epilepsy: A Phase 2 Study
Abstract
Background and objectives: To explore efficacy/safety of natalizumab, a humanized monoclonal anti-α4-integrin antibody, as adjunctive therapy in adults with drug-resistant focal epilepsy.
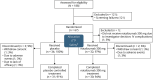
Methods: Participants with ≥6 seizures during the 6-week baseline period were randomized 1:1 to receive natalizumab 300 mg IV or placebo every 4 weeks for 24 weeks. Primary efficacy outcome was change from baseline in log-transformed seizure frequency, with a predefined threshold for therapeutic success of 31% relative reduction in seizure frequency over the placebo group. Countable seizure types were focal aware with motor signs, focal impaired awareness, and focal to bilateral tonic-clonic. Secondary efficacy endpoints/safety were also assessed.
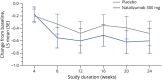
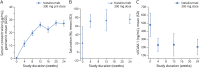
Results: Of 32 and 34 participants dosed in the natalizumab 300 mg and placebo groups, 30 (94%) and 31 (91%) completed the placebo-controlled treatment period, respectively (one participant was randomized to receive natalizumab but not dosed due to IV complications). Estimated relative change in seizure frequency of natalizumab over placebo was -14.4% (95% confidence interval [CI] -46.1%-36.1%; p = 0.51). The proportion of participants with ≥50% reduction from baseline in seizure frequency was 31.3% for natalizumab and 17.6% for placebo (odds ratio 2.09, 95% CI 0.64-6.85; p = 0.22). Adverse events were reported in 24 (75%) and 22 (65%) participants receiving natalizumab vs placebo.
Discussion: Although the threshold to demonstrate efficacy was not met, there were no unexpected safety findings and further exploration of possible anti-inflammatory therapies for drug-resistant epilepsy is warranted.
Trial registration information: The ClinicalTrials.gov registration number is NCT03283371.
Classification of evidence: This study provides Class I evidence that IV natalizumab every 4 weeks, compared to placebo, did not significantly change seizure frequency in adults with drug-resistant epilepsy. The study lacked the precision to exclude an important effect of natalizumab.
© 2021 American Academy of Neurology.
Figures
Comment in
-
Inflammation as a Target for Epilepsy Therapy: The Case of Natalizumab.Neurology. 2021 Nov 2;97(18):845-846. doi: 10.1212/WNL.0000000000012768. Epub 2021 Sep 14. Neurology. 2021. PMID: 34521688 No abstract available.
References
-
- Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(suppl 2):S2-S12. - PubMed
-
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5(5):553-564. - PubMed
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. - PubMed
-
- Nadkarni S, LaJoie J, Devinsky O. Current treatments of epilepsy. Neurology. 2005;64(12 suppl 3):S2-S11. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
