Biological Therapy in Noninfectious Pediatric Uveitis: A Systematic Review
- PMID: 34522080
- PMCID: PMC8434856
- DOI: 10.2147/OPTH.S322445
Biological Therapy in Noninfectious Pediatric Uveitis: A Systematic Review
Abstract
Purpose: Noninfectious pediatric uveitis is a potentially blinding disease often associated with systemic conditions. In cases of chronic anterior uveitis without adequate response to steroids and immunosuppressants, biological response modifiers would be viable therapeutic options. Still, evidence is lacking on the safety of the long-term use of these drugs in children. Therefore, this study aimed to evaluate the efficacy and safety of biological therapy to treat noninfectious pediatric uveitis.
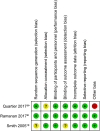
Methods: A systematic review was performed to identify original studies involving biological therapy for children diagnosed with noninfectious uveitis. Quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) classification system.
Results: Nine studies involving 526 children were eligible. Adalimumab was superior to placebo in reducing inflammatory activity (risk ratio (RR) 3.21 [95% confidence interval (CI) 1.65-6.27]; P = 0.0006; I2 = 0%) and steroid use (RR 2.27 [95% CI 1.03-4.99]; P = 0.04; I2 = 0%, low-certainty evidence). There was no difference between adalimumab and placebo in the occurrence of systemic adverse events (RR 2.51 [95% CI 0.74-8.54]; P = 0.14; I2 = 48%) and local events (RR 1.15 [95% CI 0.46-2.88]; P= 0.76; I2 = 1%). There was no difference between adalimumab and infliximab in response to treatment (RR 1.18 [95% CI 0.69-2.03]; P= 0.55; I2 = 91%, very low-certainty evidence) and in the occurrence of adverse effects (RR 0.84 [95% CI 0.41-1.73]; P= 0.64; I2 = 18%, low-certainty evidence).
Conclusion: There is low to very-low evidence that biological therapy is effective and safe in managing noninfectious pediatric uveitis. Future large randomized trials may provide more substantial evidence to confirm these results.
Keywords: biological therapy; children; systematic review; uveitis.
© 2021 Norcia et al.
Conflict of interest statement
None of the authors has any potential conflict of interest to disclose.
Figures


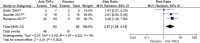

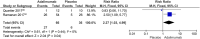

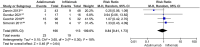





References
Publication types
LinkOut - more resources
Full Text Sources

