An Integrated Analysis of Network Pharmacology and Experimental Validation to Reveal the Mechanism of Chinese Medicine Formula Naotaifang in Treating Cerebral Ischemia-Reperfusion Injury
- PMID: 34522084
- PMCID: PMC8434864
- DOI: 10.2147/DDDT.S328837
An Integrated Analysis of Network Pharmacology and Experimental Validation to Reveal the Mechanism of Chinese Medicine Formula Naotaifang in Treating Cerebral Ischemia-Reperfusion Injury
Abstract
Background: Cerebral ischemia-reperfusion injury (CIRI) is a crucial factor leading to a poor prognosis for ischemic stroke patients. As a novel Chinese medicine formula, Naotaifang (NTF) was proven to exhibit a neuroprotective effect against ischemic stroke, clinically, and to alleviate CIRI in animals. However, the mechanisms underlying the beneficial effect have not been fully elucidated.
Methods: In this study, we combined a network pharmacology approach and an in vivo experiment to explore the specific effects and underlying mechanisms of NTF in the treatment of ischemia-reperfusion injury. A research strategy based on network pharmacology, combining target prediction, network construction, gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, and molecular docking was used to predict the targets of NTF in treating the ischemic stroke and CIRI. On the other hand, we used HPLC and HRMS to identify biologically active components of NTF. Middle cerebral artery occlusion models in rats were utilized to evaluate the effect and the underlying mechanisms of NTF against CIRI after ischemic stroke.
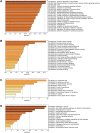
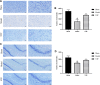
Results: Network pharmacology analysis revealed 43 potential targets and 14 signaling pathways for the treatment of NTF against CIRI after ischemic stroke. Functional enrichment analysis showed that a STAT3/PI3K/AKT signaling pathway serves as the target for in vivo experimental study validation. The results of animal experiments showed that NTF significantly alleviated CIRI by decreasing neurological score, infarct volume, numbers of apoptotic neuronal cells, increasing density of dendritic spines and survival of neurons. Furthermore, NTF could increase the expression of p-STAT3, PI3K, p-AKT. In addition, the detection of apoptosis-related factors showed that the NTF could raise the expression of Bcl-2 and reduce the expression of Bax.
Conclusion: This network pharmacological and experimental study indicated that NTF, as a therapeutic candidate for the management of CIRI following ischemic stroke, may exert a protective effect through the STAT3/PI3K/AKT signaling pathway.
Keywords: STAT3/PI3K/AKT signaling pathway; cerebral ischemia-reperfusion injury; molecular docking; network pharmacology; stroke.
© 2021 Yang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest for this work.
Figures














References
-
- Wu SM, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. - PubMed
-
- Rabinstein AA. Treatment of acute ischemic stroke. Contin Lifelong Learn Neurol. 2017;23(1):62–81. - PubMed
-
- Lahr MMH, Luijckx GJ, Vroomen PCAJ, Van Der Zee DJ, Buskens E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke. 2012;43(5):1336–1340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

