A case series of emtricitabine-induced pure red cell aplasia
- PMID: 34522429
- PMCID: PMC8424770
- DOI: 10.4102/sajhivmed.v22i1.1271
A case series of emtricitabine-induced pure red cell aplasia
Abstract
Background: Anaemia is common in patients with retroviral disease. New or worsening anaemia after initiation of antiretroviral (ARV) treatment has a broad differential diagnosis.
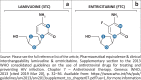
Objectives: We describe six patients who developed transfusion-dependent anaemia on first-line therapy (tenofovir, emtricitabine and efavirenz) and, by exclusion, implicated emtricitabine in the aetiology of the anaemia.
Method: We conducted a retrospective chart review of patients seen at the Infectious Diseases specialist clinic at King Edward VIII Hospital in KwaZulu-Natal between 2014 and 2016. We focused on patients with isolated, refractory and transfusion-dependent anaemia occurring after initiation of ARVs, in whom bone marrow biopsies were consistent with pure red cell aplasia (PRCA) without an identifiable secondary cause.
Results: All the patients were female, with a median (range) age and baseline CD4 cell count of 42.5 (23-61) years and 237 (83-329) cells/mm3, respectively. Before presenting with symptomatic anaemia, the duration on emtricitabine was 4.5 (2-8) months. At presentation, all patients had an HIV viral load of < 1000 copies/mL and a CD4 cell count of 314 (213-389) cells/mm3. The median time to recovery following the discontinuation of emtricitabine was 2 (1-4) months. After a median of 12 months, all patients were successfully rechallenged with emtricitabine and remained well for a follow-up period of 24 (7-36) months.
Conclusion: This study provides strong circumstantial evidence that emtricitabine plays an important role in the pathogenesis of reversible PRCA. The mechanisms through which emtricitabine induces PRCA remain unclear and require further study.
Keywords: adverse drug reaction; anaemia; antiretroviral; drug induced; emtricitabine; pure red cell aplasia; rare drug toxicity.
© 2021. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures
References
-
- Hester EK, PeacockJE. Jr. Profound and unanticipated anemia with lamivudine-zidovudine combination therapy in zidovudine-experienced patients with HIV infection. AIDS. 1998;12(4):439–440. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials